Abstract
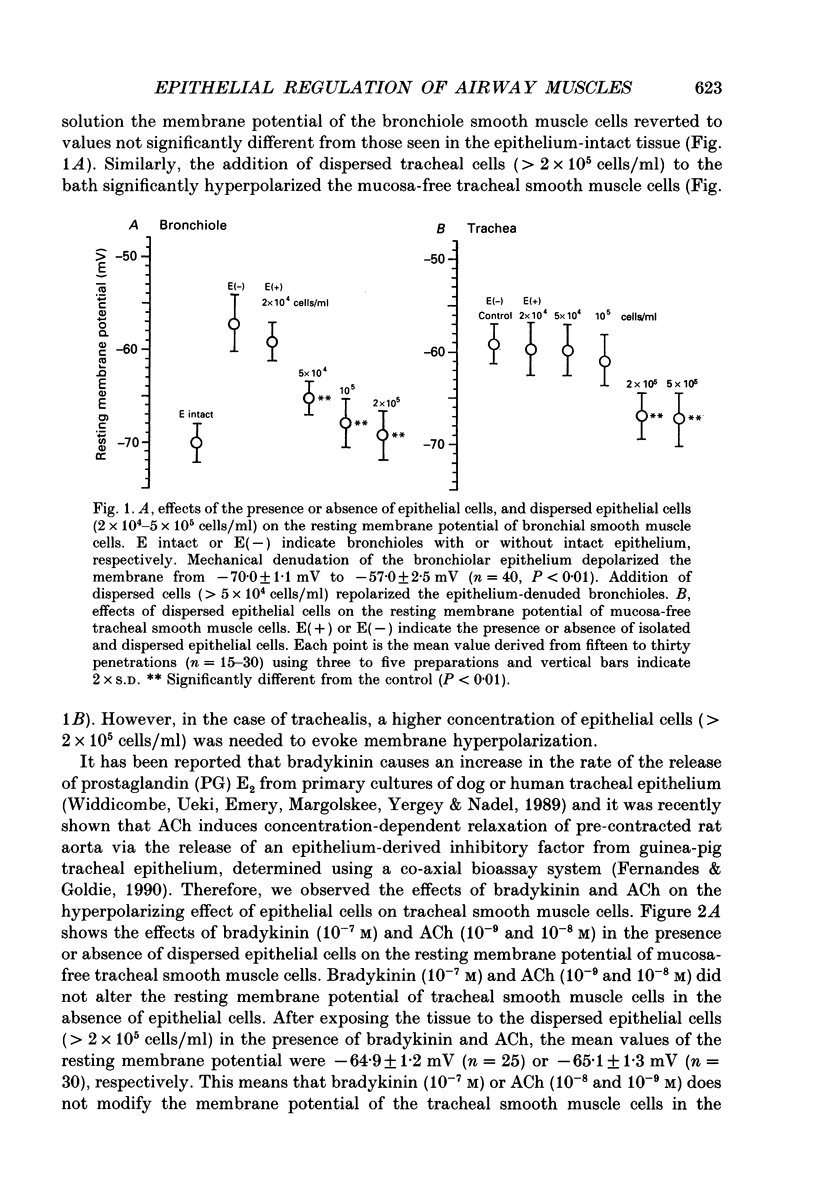
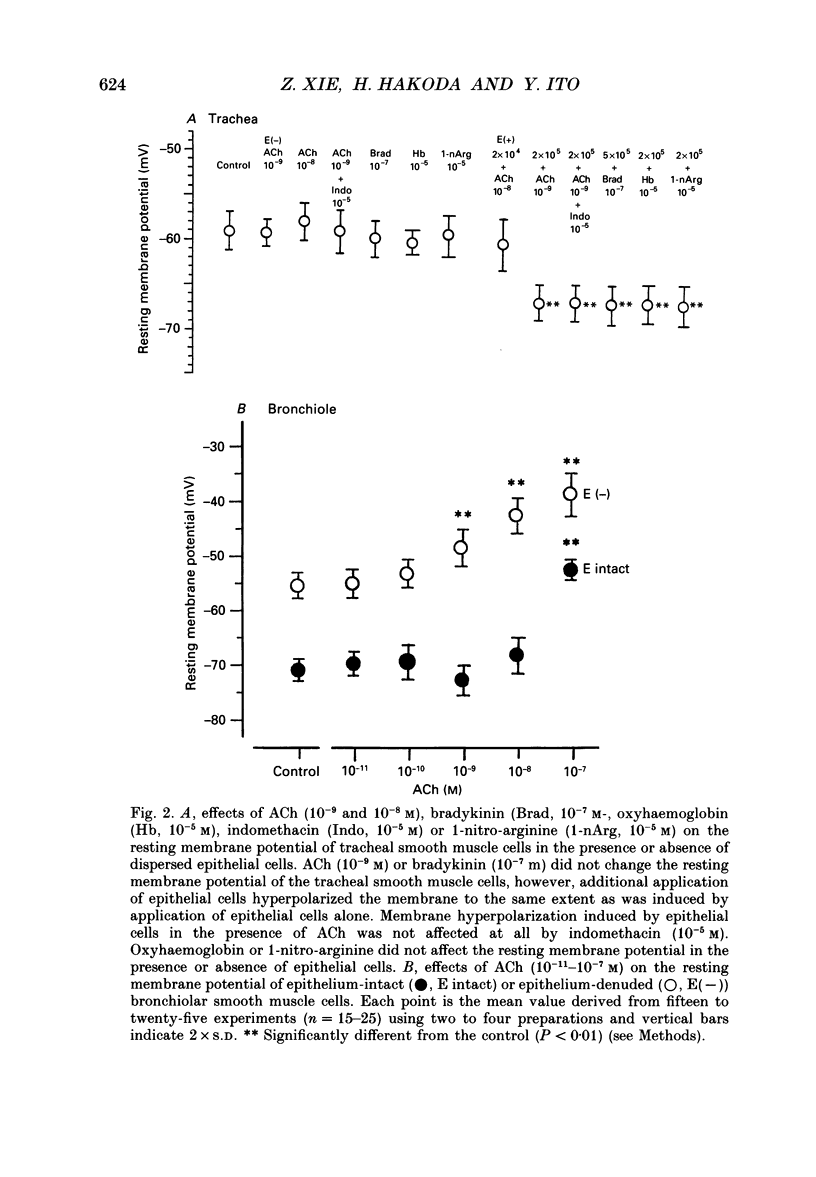
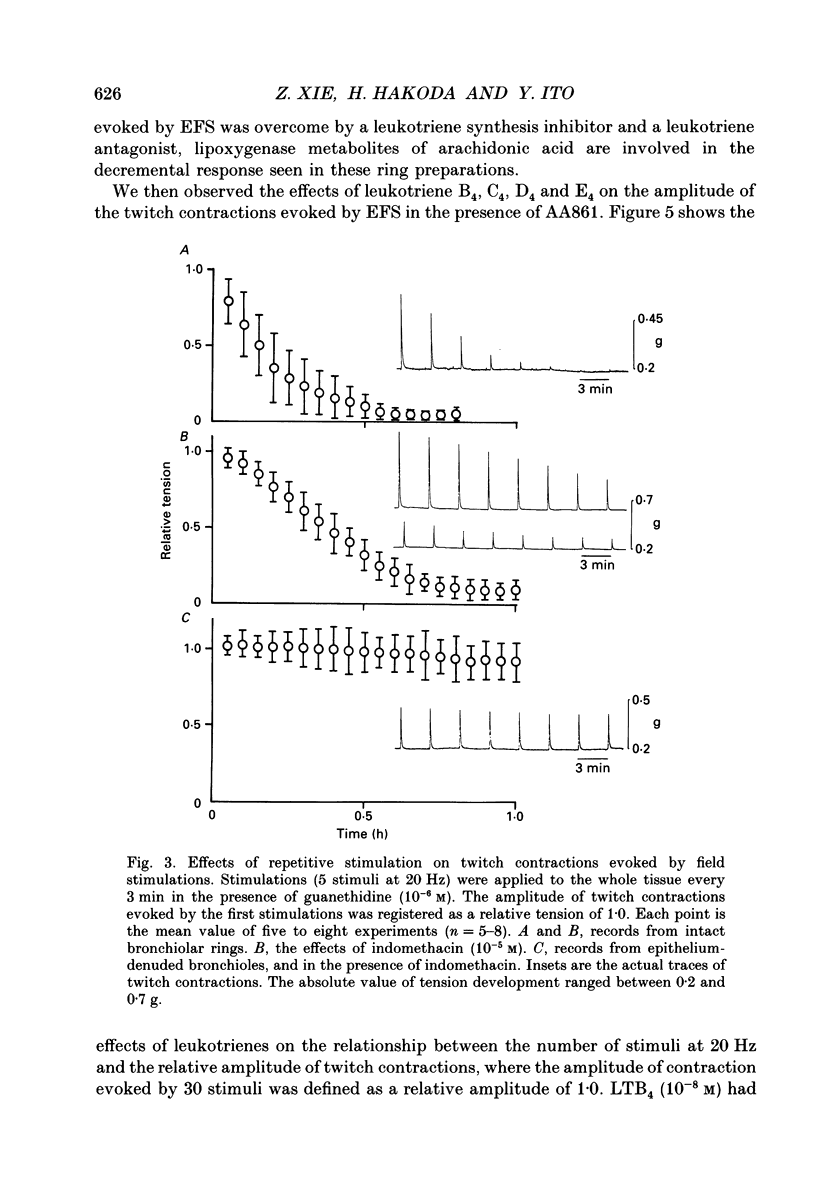
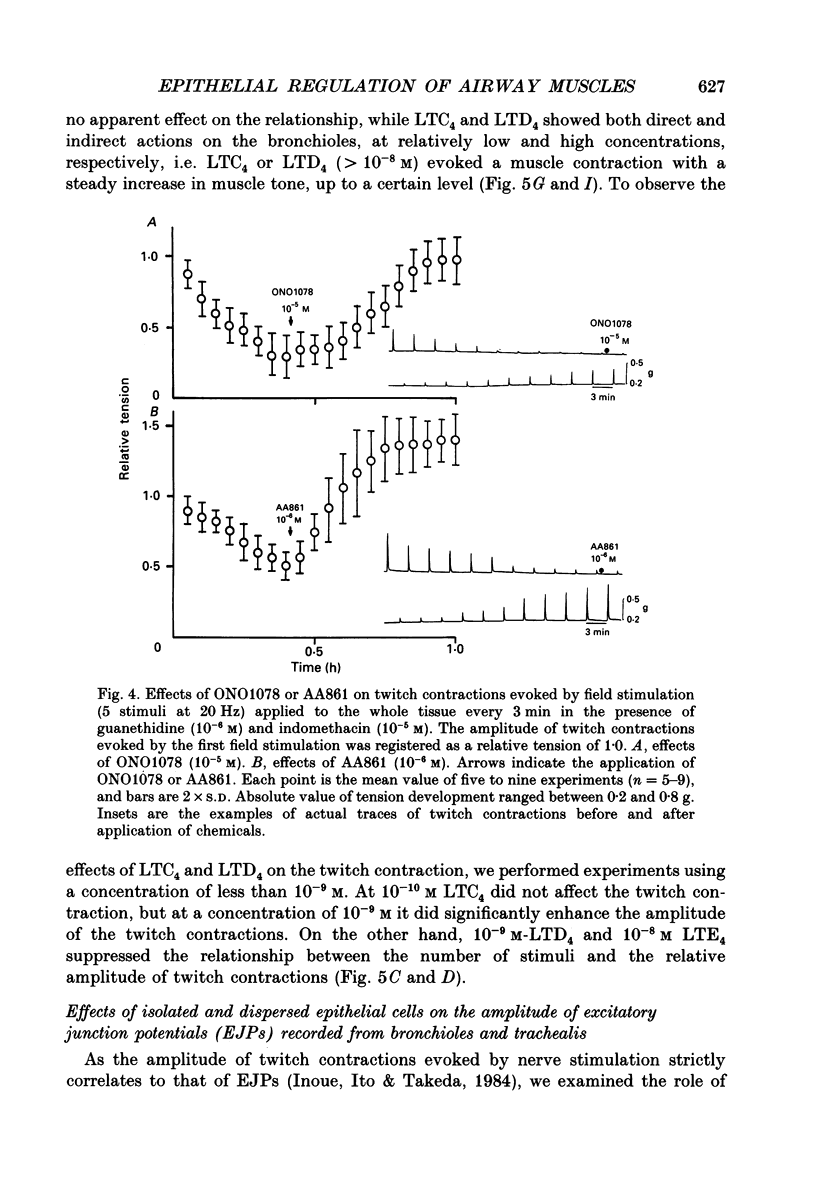
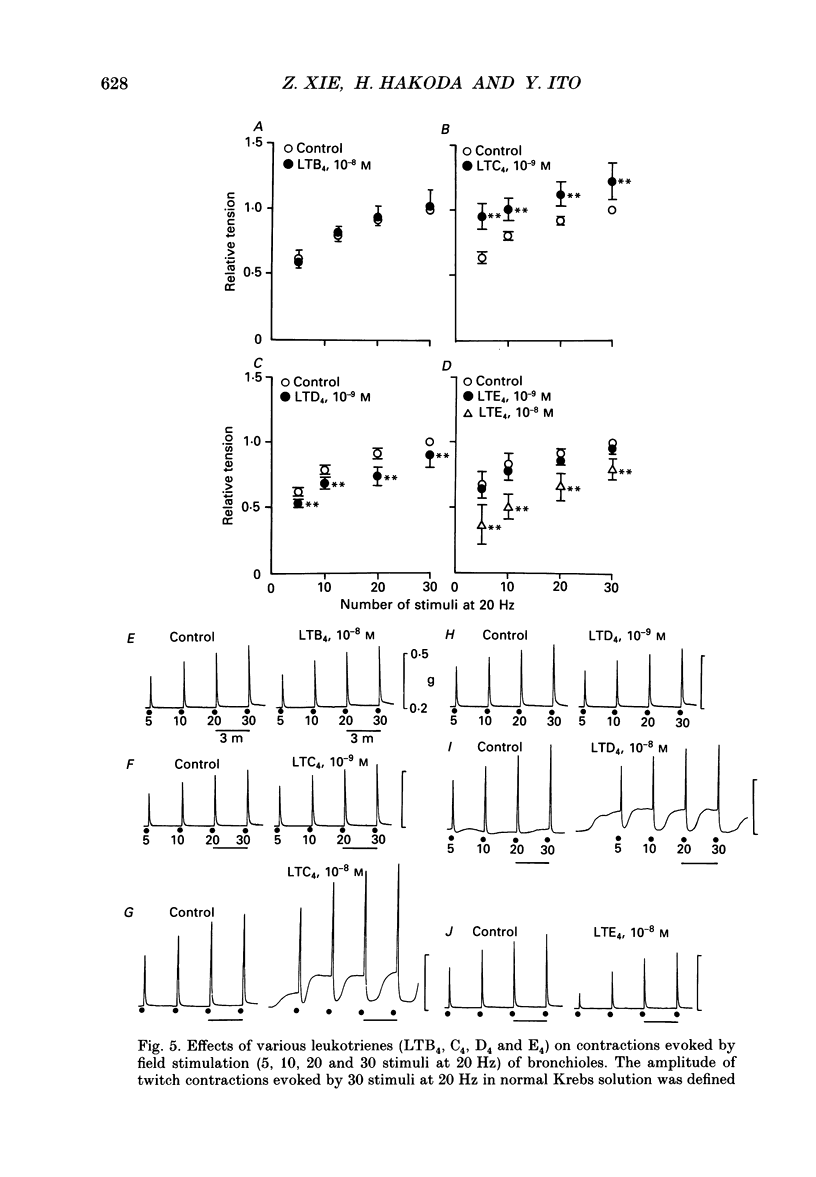
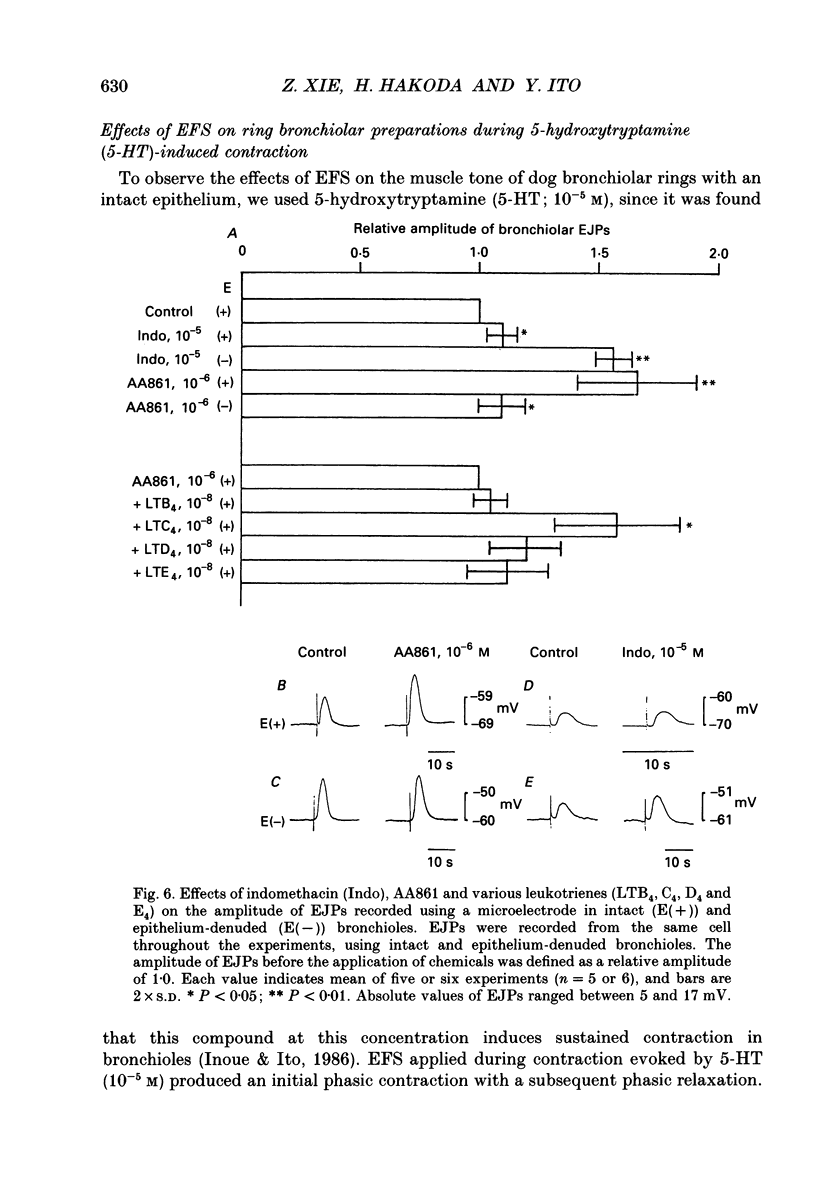
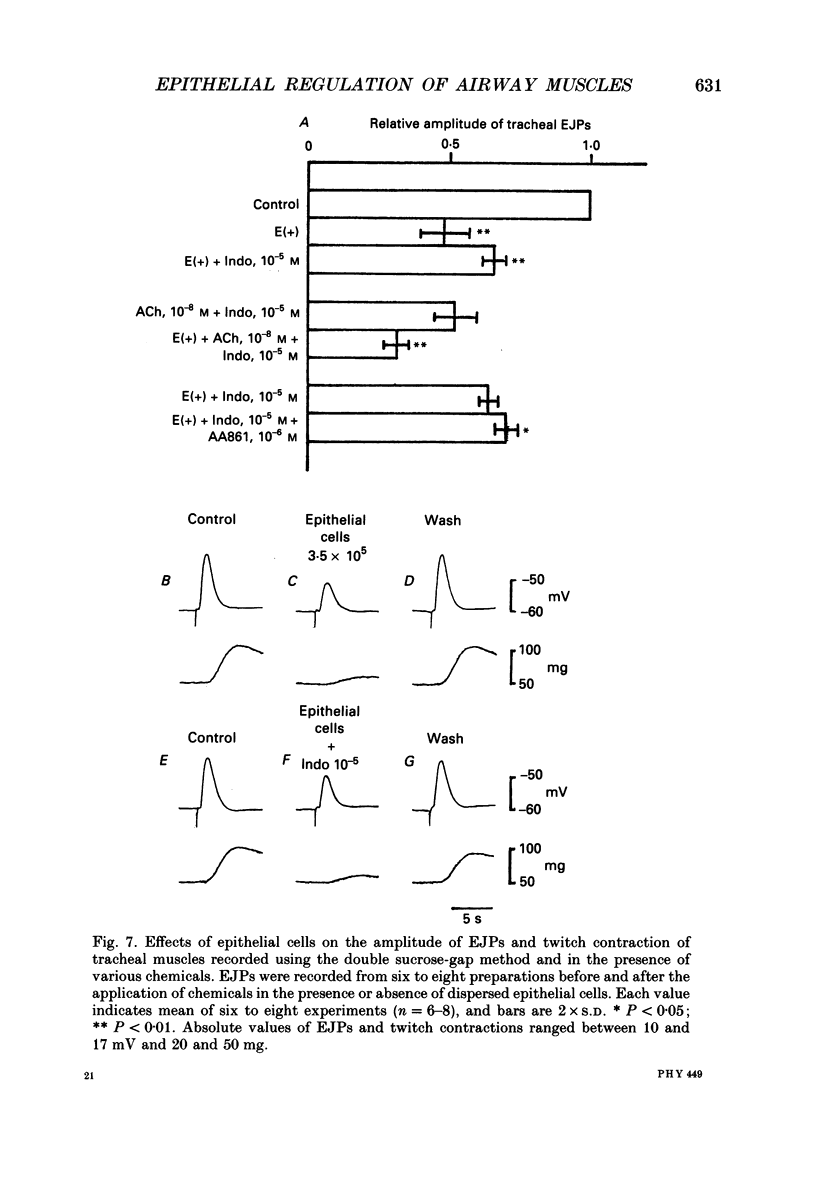
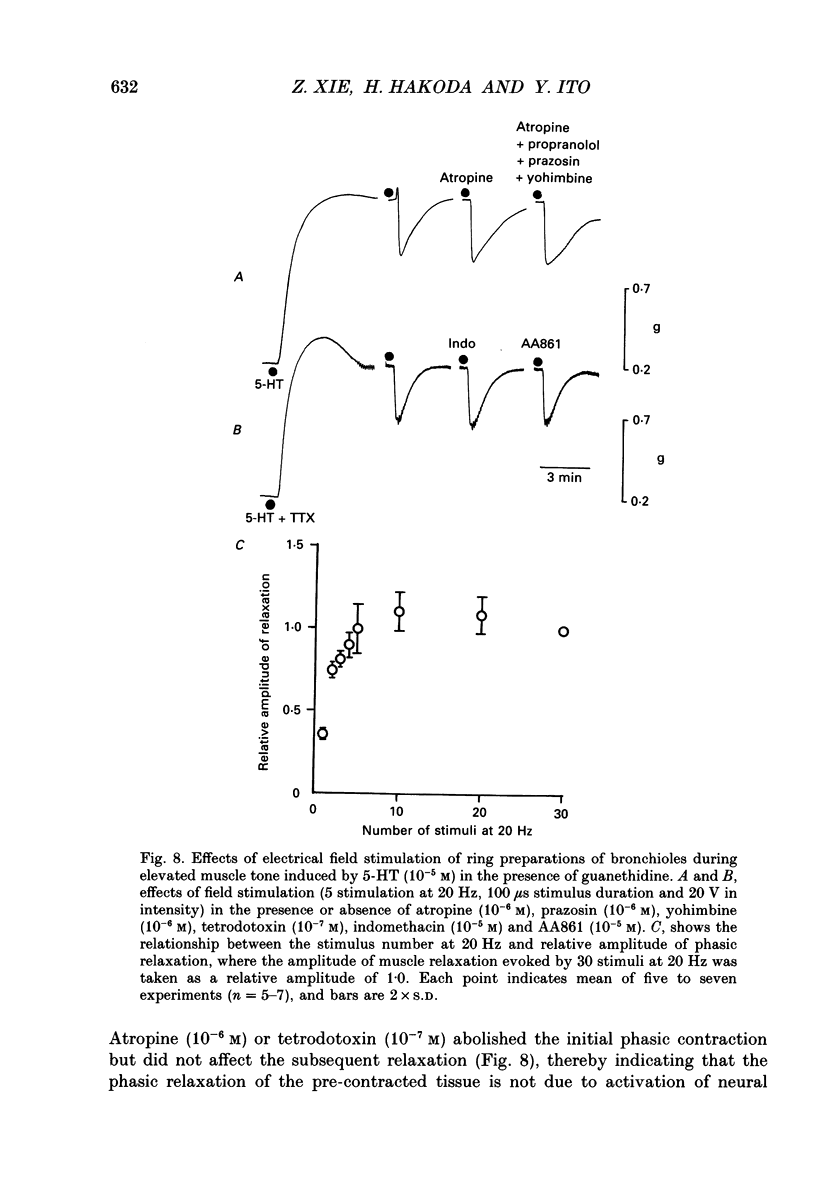
1. The effects of epithelial cells were investigated on resting membrane potential and neuro-effector transmission in smooth muscle cells of the dog tracheal and bronchiolar tissues. 2. The mean value of the resting membrane potential of the epithelium-intact bronchiolar smooth muscle cells of the dog was--70.0 +/- 1.1 mV (+/- S.D., n = 40) and mechanical denudation of the epithelial layer depolarized the membrane to -57.0 +/- 2.5 mV (+/- S.D., n = 40). Application of isolated and dispersed epithelial cells (greater than 2 x 10(5) cells/ml) to the perfusing solution repolarized the membrane of epithelium-denuded bronchiolar smooth muscle cells to -67.0 +/- 2.7 mV (+/- S.D., n = 20). The mean resting membrane potential of the mucosa-free tracheal smooth muscle cells was -59.1 +/- 1.4 mV (+/- S.D., n = 50), and application of isolated and dispersed cells (greater than 2 x 10(5) cells/ml) hyperpolarized the membrane to -67.2 +/- 1.8 mV (+/- S.D., n = 50). These repolarizing actions were not modified by indomethacin (10(-5) M). 3. In the epithelium-denuded bronchioles, ACh (greater than 10(-9) M) dose-dependently depolarized the smooth muscle cells, while in the epithelium-intact bronchioles, ACh (10(-11) - 10(-8) M) did not affect the resting membrane potential. At a concentration of 10(-7) M, ACh significantly depolarized the membrane. 4. Electrical field stimulation (EFS; 50 microseconds in duration and about 10-20 V in strength) applied to ring preparations of the bronchioles evoked twitch-like contractions (hereafter referred as twitch contraction), and size of the twitch contractions gradually and continuously decreased in the presence or absence of indomethacin (10(-5) M) and guanethidine (10(-6) M). When similar experiments were performed using epithelium-denuded bronchiolar ring preparations, in no case was there a prominent reduction in the amplitude of the twitch contractions in the presence of indomethacin and guanethidine. 5. The decremental response of the twitch contraction observed in the epithelium-intact bronchioles was overcome by application of the leukotriene synthesis inhibitor AA861 (10(-6) M) and the leukotriene antagonist ONO1078 (10(-5) M). 6. Leukotrienes C4 and D4 (LTC4 and LTD4, greater than 10(-8) M) evoked muscle contraction with a steady increase in muscle tone, up to a certain level. However, at 10(-9) M, LTC4 increased and LTD4 decreased the amplitude of the twitch contractions evoked by EFS in the epithelium-intact bronchioles.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes P. J., Cuss F. M., Palmer J. B. The effect of airway epithelium on smooth muscle contractility in bovine trachea. Br J Pharmacol. 1985 Nov;86(3):685–691. doi: 10.1111/j.1476-5381.1985.tb08946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett K., Jacoby D. B., Nadel J. A., Lazarus S. C. The effects of epithelial cell supernatant on contractions of isolated canine tracheal smooth muscle. Am Rev Respir Dis. 1988 Oct;138(4):780–783. doi: 10.1164/ajrccm/138.4.780. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Lang R. J., Takewaki T. Mechanisms of action of noradrenaline and carbachol on smooth muscle of guinea-pig anterior mesenteric artery. J Physiol. 1984 Jun;351:549–572. doi: 10.1113/jphysiol.1984.sp015262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. F., Suzuki H. Calcium dependency of the endothelium-dependent hyperpolarization in smooth muscle cells of the rabbit carotid artery. J Physiol. 1990 Feb;421:521–534. doi: 10.1113/jphysiol.1990.sp017959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Suzuki H., Weston A. H. Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br J Pharmacol. 1988 Dec;95(4):1165–1174. doi: 10.1111/j.1476-5381.1988.tb11752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming G. Airway morphology and its consequences. Bull Physiopathol Respir (Nancy) 1972 May-Jun;8(3):527–533. [PubMed] [Google Scholar]
- Danser A. H., van den Ende R., Lorenz R. R., Flavahan N. A., Vanhoutte P. M. Prejunctional beta 1-adrenoceptors inhibit cholinergic transmission in canine bronchi. J Appl Physiol (1985) 1987 Feb;62(2):785–790. doi: 10.1152/jappl.1987.62.2.785. [DOI] [PubMed] [Google Scholar]
- Farley J. M., Miles P. R. Role of depolarization in acetylcholine-induced contractions of dog trachealis muscle. J Pharmacol Exp Ther. 1977 Apr;201(1):199–205. [PubMed] [Google Scholar]
- Fernandes L. B., Goldie R. G. Pharmacological evaluation of a guinea-pig tracheal epithelium-derived inhibitory factor (EpDIF). Br J Pharmacol. 1990 Jul;100(3):614–618. doi: 10.1111/j.1476-5381.1990.tb15855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes L. B., Paterson J. W., Goldie R. G. Co-axial bioassay of a smooth muscle relaxant factor released from guinea-pig tracheal epithelium. Br J Pharmacol. 1989 Jan;96(1):117–124. doi: 10.1111/j.1476-5381.1989.tb11791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzell R. A., Welsh M. J., Smith P. L. Hormonal control of chloride secretion by canine tracheal epithelium: an electrophysiologic analysis. Ann N Y Acad Sci. 1981;372:558–570. doi: 10.1111/j.1749-6632.1981.tb15506.x. [DOI] [PubMed] [Google Scholar]
- Goldie R. G., Fernandes L. B., Farmer S. G., Hay D. W. Airway epithelium-derived inhibitory factor. Trends Pharmacol Sci. 1990 Feb;11(2):67–70. doi: 10.1016/0165-6147(90)90320-8. [DOI] [PubMed] [Google Scholar]
- Güc M. O., Ilhan M., Kayaalp S. O. The rat anococcygeus muscle is a convenient bioassay organ for the airway epithelium-derived relaxant factor. Eur J Pharmacol. 1988 Apr 13;148(3):405–409. doi: 10.1016/0014-2999(88)90119-7. [DOI] [PubMed] [Google Scholar]
- Hay D. W., Farmer S. G., Raeburn D., Muccitelli R. M., Wilson K. A., Fedan J. S. Differential effects of epithelium removal on the responsiveness of guinea-pig tracheal smooth muscle to bronchoconstrictors. Br J Pharmacol. 1987 Oct;92(2):381–388. doi: 10.1111/j.1476-5381.1987.tb11334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisayama T., Takayanagi I., Nakazato F., Hirano K. Epithelium selectively controls hypersensitization of the response of smooth muscle to leukotriene D4 by endogenous prostanoid(s) in guinea-pig trachea. Naunyn Schmiedebergs Arch Pharmacol. 1988 Mar;337(3):296–300. doi: 10.1007/BF00168842. [DOI] [PubMed] [Google Scholar]
- Ilhan M., Sahin I. Tracheal epithelium releases a vascular smooth muscle relaxant factor: demonstration by bioassay. Eur J Pharmacol. 1986 Nov 19;131(2-3):293–296. doi: 10.1016/0014-2999(86)90586-8. [DOI] [PubMed] [Google Scholar]
- Inoue T., Ito Y. Characteristics of neuro-effector transmission in the smooth muscle layer of dog bronchiole and modifications by autacoids. J Physiol. 1986 Jan;370:551–565. doi: 10.1113/jphysiol.1986.sp015950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T., Ito Y. Pre- and post-junctional actions of prostaglandin I2, carbocyclic thromboxane A2 and leukotriene C4 in dog tracheal tissue. Br J Pharmacol. 1985 Feb;84(2):289–298. doi: 10.1111/j.1476-5381.1985.tb12913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T., Ito Y., Takeda K. Prostaglandin-induced inhibition of acetylcholine release from neuronal elements of dog tracheal tissue. J Physiol. 1984 Apr;349:553–570. doi: 10.1113/jphysiol.1984.sp015173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Inoue T. Contracture and change in membrane potential produced by sodium removal in the dog trachea and bronchiole. J Appl Physiol (1985) 1989 Nov;67(5):2078–2086. doi: 10.1152/jappl.1989.67.5.2078. [DOI] [PubMed] [Google Scholar]
- Ito Y. Pre- and post-junctional actions of procaterol, a beta 2-adrenoceptor stimulant, on dog tracheal tissue. Br J Pharmacol. 1988 Sep;95(1):268–274. doi: 10.1111/j.1476-5381.1988.tb16573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y. Prejunctional control of excitatory neuroeffector transmission by prostaglandins in the airway smooth muscle tissue. Am Rev Respir Dis. 1991 Mar;143(3 Pt 2):S6–10. doi: 10.1164/ajrccm/143.3_Pt_2.S6. [DOI] [PubMed] [Google Scholar]
- Ito Y., Tajima K. Actions of indomethacin and prostaglandins on neuro-effector transmission in the dog trachea. J Physiol. 1981;319:379–392. doi: 10.1113/jphysiol.1981.sp013915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Yoshitomi T. Autoregulation of acetylcholine release from vagus nerve terminals through activation of muscarinic receptors in the dog trachea. Br J Pharmacol. 1988 Mar;93(3):636–646. doi: 10.1111/j.1476-5381.1988.tb10321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori K., Lorenz R. R., Vanhoutte P. M. Nitric oxide, ACh, and electrical and mechanical properties of canine arterial smooth muscle. Am J Physiol. 1988 Jul;255(1 Pt 2):H207–H212. doi: 10.1152/ajpheart.1988.255.1.H207. [DOI] [PubMed] [Google Scholar]
- Komori K., Suzuki H. Electrical responses of smooth muscle cells during cholinergic vasodilation in the rabbit saphenous artery. Circ Res. 1987 Oct;61(4):586–593. doi: 10.1161/01.res.61.4.586. [DOI] [PubMed] [Google Scholar]
- Munakata M., Mitzner W., Menkes H. Osmotic stimuli induce epithelial-dependent relaxation in the guinea pig trachea. J Appl Physiol (1985) 1988 Jan;64(1):466–471. doi: 10.1152/jappl.1988.64.1.466. [DOI] [PubMed] [Google Scholar]
- Nagao T., Suzuki H. Non-neural electrical responses of smooth muscle cells of the rabbit basilar artery to electrical field stimulation. Jpn J Physiol. 1987;37(3):497–513. doi: 10.2170/jjphysiol.37.497. [DOI] [PubMed] [Google Scholar]
- Nijkamp F. P., Folkerts G. Reversal of arachidonic acid-induced guinea-pig tracheal relaxation into contraction after epithelium removal. Eur J Pharmacol. 1986 Nov 19;131(2-3):315–316. doi: 10.1016/0014-2999(86)90591-1. [DOI] [PubMed] [Google Scholar]
- Russell J. A. Noradrenergic inhibitory innervation of canine airways. J Appl Physiol Respir Environ Exerc Physiol. 1980 Jan;48(1):16–22. doi: 10.1152/jappl.1980.48.1.16. [DOI] [PubMed] [Google Scholar]
- Smedegård G., Hedqvist P., Dahlén S. E., Revenäs B., Hammarström S., Samuelsson B. Leukotriene C4 affects pulmonary and cardiovascular dynamics in monkey. Nature. 1982 Jan 28;295(5847):327–329. doi: 10.1038/295327a0. [DOI] [PubMed] [Google Scholar]
- Souhrada M., Klein J. J., Berend N., Souhrada J. F. Topographical differences in the physiological response of canine airway smooth muscle. Respir Physiol. 1983 May;52(2):245–258. doi: 10.1016/0034-5687(83)90009-9. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Morita K., Kuriyama H. Innervation and properties of the smooth muscle of the dog trachea. Jpn J Physiol. 1976;26(3):303–320. doi: 10.2170/jjphysiol.26.303. [DOI] [PubMed] [Google Scholar]
- Tschirhart E., Frossard N., Bertrand C., Landry Y. Arachidonic acid metabolites and airway epithelium-dependent relaxant factor. J Pharmacol Exp Ther. 1987 Oct;243(1):310–316. [PubMed] [Google Scholar]
- Tschirhart E., Landry Y. Airway epithelium releases a relaxant factor: demonstration with substance P. Eur J Pharmacol. 1986 Dec 2;132(1):103–104. doi: 10.1016/0014-2999(86)90020-8. [DOI] [PubMed] [Google Scholar]
- Ullman A., Löfdahl C. G., Svedmyr N., Skoogh B. E. Nerve stimulation releases mucosa-derived inhibitory factors, both prostanoids and nonprostanoids, in isolated ferret trachea. Am Rev Respir Dis. 1990 Mar;141(3):748–751. doi: 10.1164/ajrccm/141.3.748. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. H., Ueki I. F., Emery D., Margolskee D., Yergey J., Nadel J. A. Release of cyclooxygenase products from primary cultures of tracheal epithelia of dog and human. Am J Physiol. 1989 Dec;257(6 Pt 1):L361–L365. doi: 10.1152/ajplung.1989.257.6.L361. [DOI] [PubMed] [Google Scholar]


