Abstract
1. Histamine-stimulated mobilization of intracellular Ca2+ stores was monitored in intact and permeabilized populations of HeLa cells using both the fluorescent Ca2+ indicator Fura-2 and 45Ca2+ measurements. Digital video imaging of Fura-2-loaded cells was used to measure the intracellular calcium concentration ([Ca2+]i) of single cells. 2. In populations of HeLa cells, histamine caused a concentration-dependent increase in cytoplasmic [Ca2+]. The initial transient increase was independent of extracellular Ca2+ (Ca2+o) and was followed by a sustained increase that was abolished by removal of Ca2+o. 3. In Ca(2+)-free medium ([Ca2+]o < 1 microM), a maximal histamine concentration (25 microM) caused a transient increase in [Ca2+]i, and a subsequent challenge with histamine failed to evoke a further response indicating that the inositol 1,4,5-trisphosphate (InsP3)-sensitive Ca2+ stores had been completely emptied. Lower concentrations of histamine (0.5-10 microM) caused smaller, concentration-dependent increases in [Ca2+]i that were also transient. After exposure to these low histamine concentrations, where [Ca2+]i returned to baseline within 2 min, addition of a higher histamine concentration evoked a further increase in [Ca2+]i. The second increase in [Ca2+]i was inversely proportional to the increase caused by the first exposure to histamine, indicating that Ca2+ released in the initial response was not substantially resequestered into histamine-sensitive stores. 4. Single HeLa cells challenged with low concentrations of histamine in Ca(2+)-free medium responded with transient increases in [Ca2+]i, but individual cells differed in their sensitivity with 51% of cells responding to 1 microM, and 98% responding to 25 microM-histamine. 5. When single cells in Ca(2+)-free medium were challenged with stepwise increases in histamine concentration, they responded to each step with a transient [Ca2+]i increase after which [Ca2+]i returned to baseline within 1 min. Prolonging the interval between histamine additions by up to 25 min did not affect the [Ca2+]i increase evoked by a subsequent histamine addition. 6. Unidirectional 45Ca2+ efflux from saponin-permeabilized HeLa cells showed that, under conditions that prevented Ca2+ resequestration, submaximal concentrations of InsP3 rapidly emptied only a fraction of the InsP3-sensitive Ca2+ stores. The failure of low InsP3 concentrations to fully mobilize the InsP3-sensitive Ca2+ stores was not a consequence of InsP3 degradation. 7. We conclude that within single HeLa cells, intracellular Ca2+ stores are heterogeneous in their sensitivity to InsP3, and the fraction of Ca2+ stores mobilized by InsP3 increases as the InsP3 concentration increases.
Full text
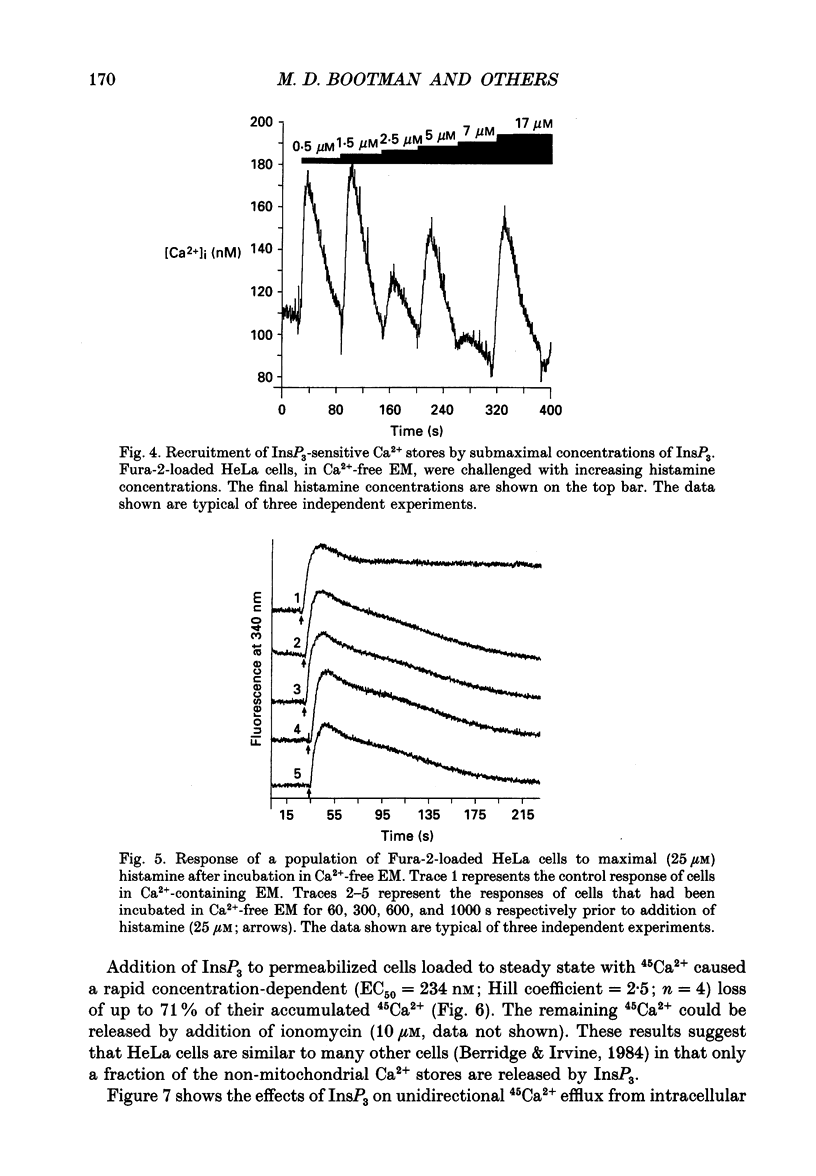
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Chadwick C. C., Saito A., Fleischer S. Isolation and characterization of the inositol trisphosphate receptor from smooth muscle. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2132–2136. doi: 10.1073/pnas.87.6.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champeil P., Combettes L., Berthon B., Doucet E., Orlowski S., Claret M. Fast kinetics of calcium release induced by myo-inositol trisphosphate in permeabilized rat hepatocytes. J Biol Chem. 1989 Oct 25;264(30):17665–17673. [PubMed] [Google Scholar]
- Finch E. A., Turner T. J., Goldin S. M. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991 Apr 19;252(5004):443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Yoshikawa S., Miyawaki A., Wada K., Maeda N., Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989 Nov 2;342(6245):32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Haaksma E. E., Leurs R., Timmerman H. Histamine receptors: subclasses and specific ligands. Pharmacol Ther. 1990;47(1):73–104. doi: 10.1016/0163-7258(90)90046-5. [DOI] [PubMed] [Google Scholar]
- Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol. 1990 Jun;95(6):1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F. 'Quantal' Ca2+ release and the control of Ca2+ entry by inositol phosphates--a possible mechanism. FEBS Lett. 1990 Apr 9;263(1):5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- Jacob R., Merritt J. E., Hallam T. J., Rink T. J. Repetitive spikes in cytoplasmic calcium evoked by histamine in human endothelial cells. Nature. 1988 Sep 1;335(6185):40–45. doi: 10.1038/335040a0. [DOI] [PubMed] [Google Scholar]
- Krause K. H., Simmerman H. K., Jones L. R., Campbell K. P. Sequence similarity of calreticulin with a Ca2(+)-binding protein that co-purifies with an Ins(1,4,5)P3-sensitive Ca2+ store in HL-60 cells. Biochem J. 1990 Sep 1;270(2):545–548. doi: 10.1042/bj2700545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechleiter J., Girard S., Peralta E., Clapham D. Spiral calcium wave propagation and annihilation in Xenopus laevis oocytes. Science. 1991 Apr 5;252(5002):123–126. doi: 10.1126/science.2011747. [DOI] [PubMed] [Google Scholar]
- Maeda N., Kawasaki T., Nakade S., Yokota N., Taguchi T., Kasai M., Mikoshiba K. Structural and functional characterization of inositol 1,4,5-trisphosphate receptor channel from mouse cerebellum. J Biol Chem. 1991 Jan 15;266(2):1109–1116. [PubMed] [Google Scholar]
- Meyer T. Cell signaling by second messenger waves. Cell. 1991 Feb 22;64(4):675–678. doi: 10.1016/0092-8674(91)90496-l. [DOI] [PubMed] [Google Scholar]
- Meyer T., Holowka D., Stryer L. Highly cooperative opening of calcium channels by inositol 1,4,5-trisphosphate. Science. 1988 Apr 29;240(4852):653–656. doi: 10.1126/science.2452482. [DOI] [PubMed] [Google Scholar]
- Meyer T., Stryer L. Transient calcium release induced by successive increments of inositol 1,4,5-trisphosphate. Proc Natl Acad Sci U S A. 1990 May;87(10):3841–3845. doi: 10.1073/pnas.87.10.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T., Wensel T., Stryer L. Kinetics of calcium channel opening by inositol 1,4,5-trisphosphate. Biochemistry. 1990 Jan 9;29(1):32–37. doi: 10.1021/bi00453a004. [DOI] [PubMed] [Google Scholar]
- Mignery G. A., Südhof T. C., Takei K., De Camilli P. Putative receptor for inositol 1,4,5-trisphosphate similar to ryanodine receptor. Nature. 1989 Nov 9;342(6246):192–195. doi: 10.1038/342192a0. [DOI] [PubMed] [Google Scholar]
- Mignery G. A., Südhof T. C. The ligand binding site and transduction mechanism in the inositol-1,4,5-triphosphate receptor. EMBO J. 1990 Dec;9(12):3893–3898. doi: 10.1002/j.1460-2075.1990.tb07609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiaen L., Taylor C. W., Berridge M. J. Spontaneous calcium release from inositol trisphosphate-sensitive calcium stores. Nature. 1991 Jul 18;352(6332):241–244. doi: 10.1038/352241a0. [DOI] [PubMed] [Google Scholar]
- Muallem S., Pandol S. J., Beeker T. G. Hormone-evoked calcium release from intracellular stores is a quantal process. J Biol Chem. 1989 Jan 5;264(1):205–212. [PubMed] [Google Scholar]
- O'Sullivan A. J., Cheek T. R., Moreton R. B., Berridge M. J., Burgoyne R. D. Localization and heterogeneity of agonist-induced changes in cytosolic calcium concentration in single bovine adrenal chromaffin cells from video imaging of fura-2. EMBO J. 1989 Feb;8(2):401–411. doi: 10.1002/j.1460-2075.1989.tb03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldershaw K. A., Nunn D. L., Taylor C. W. Quantal Ca2+ mobilization stimulated by inositol 1,4,5-trisphosphate in permeabilized hepatocytes. Biochem J. 1991 Sep 15;278(Pt 3):705–708. doi: 10.1042/bj2780705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Inhibition by Ca2+ of inositol trisphosphate-mediated Ca2+ liberation: a possible mechanism for oscillatory release of Ca2+. Proc Natl Acad Sci U S A. 1990 Jan;87(1):260–264. doi: 10.1073/pnas.87.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Localized all-or-none calcium liberation by inositol trisphosphate. Science. 1990 Nov 16;250(4983):977–979. doi: 10.1126/science.2237441. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Rooney T. A., Sass E. J., Thomas A. P. Agonist-induced cytosolic calcium oscillations originate from a specific locus in single hepatocytes. J Biol Chem. 1990 Jun 25;265(18):10792–10796. [PubMed] [Google Scholar]
- Supattapone S., Worley P. F., Baraban J. M., Snyder S. H. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J Biol Chem. 1988 Jan 25;263(3):1530–1534. [PubMed] [Google Scholar]
- Taylor C. W., Potter B. V. The size of inositol 1,4,5-trisphosphate-sensitive Ca2+ stores depends on inositol 1,4,5-trisphosphate concentration. Biochem J. 1990 Feb 15;266(1):189–194. doi: 10.1042/bj2660189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly B. C., Tertoolen L. G., Remorie R., Ladoux A., Verlaan I., de Laat S. W., Moolenaar W. H. Histamine as a growth factor and chemoattractant for human carcinoma and melanoma cells: action through Ca2(+)-mobilizing H1 receptors. J Cell Biol. 1990 Apr;110(4):1211–1215. doi: 10.1083/jcb.110.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves S., De Mattei M., Landfredi M., Villa A., Green N. M., MacLennan D. H., Meldolesi J., Pozzan T. Calreticulin is a candidate for a calsequestrin-like function in Ca2(+)-storage compartments (calciosomes) of liver and brain. Biochem J. 1990 Oct 15;271(2):473–480. doi: 10.1042/bj2710473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Khademazad M., Muallem S. Agonist-mediated Ca2+ release in permeabilized UMR-106-01 cells. Transport properties and generation of inositol 1,4,5-trisphosphate. J Biol Chem. 1990 Sep 5;265(25):14822–14827. [PubMed] [Google Scholar]


