Abstract
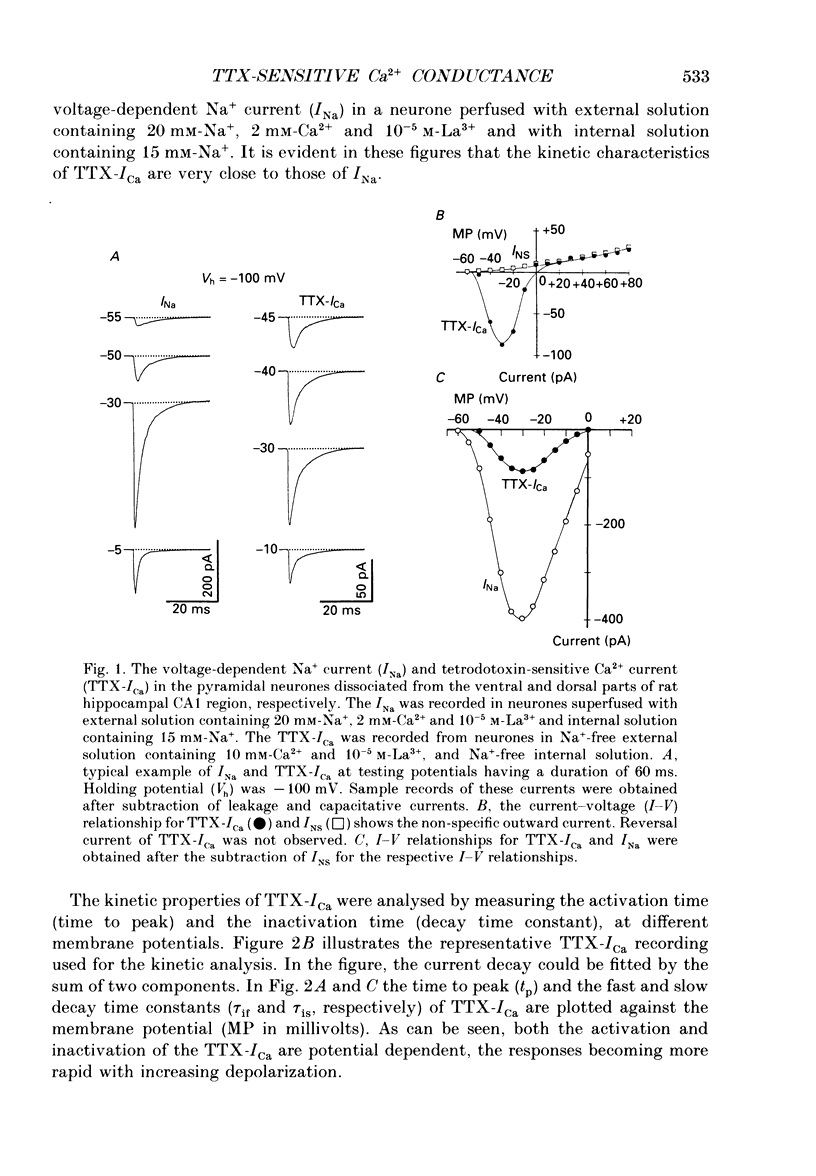
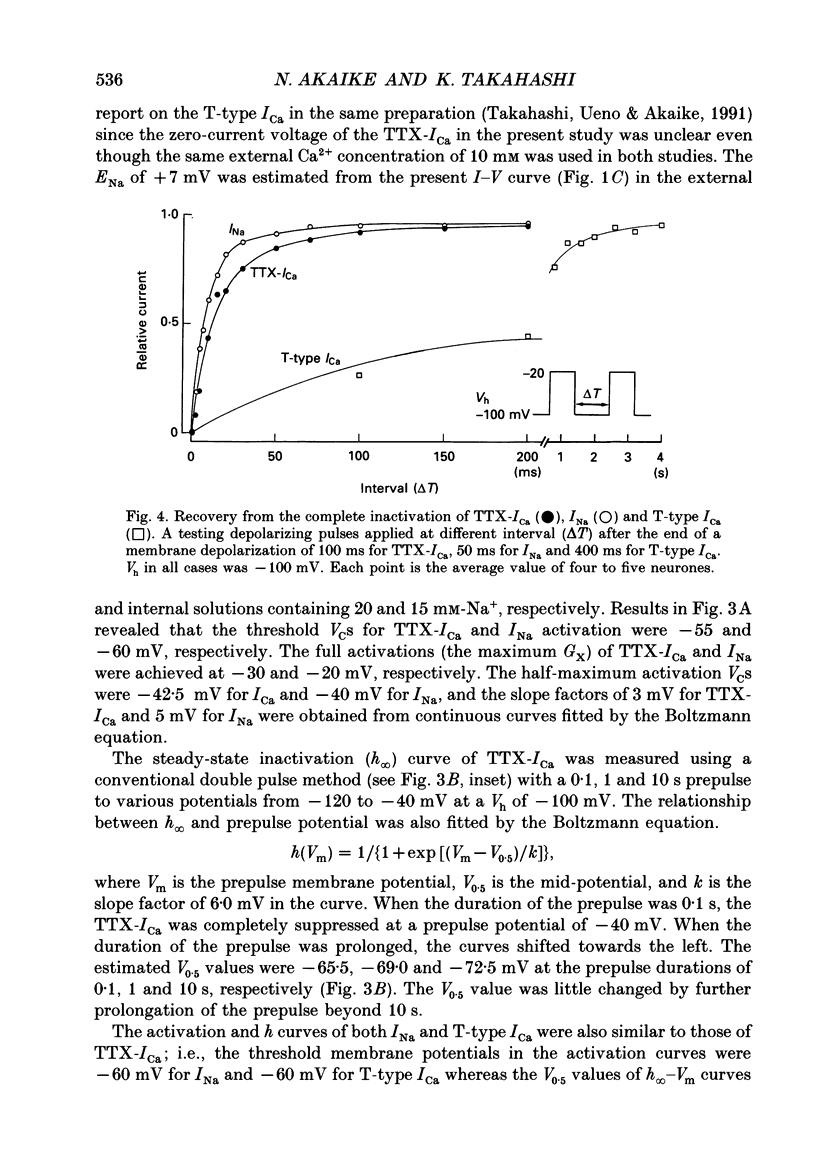
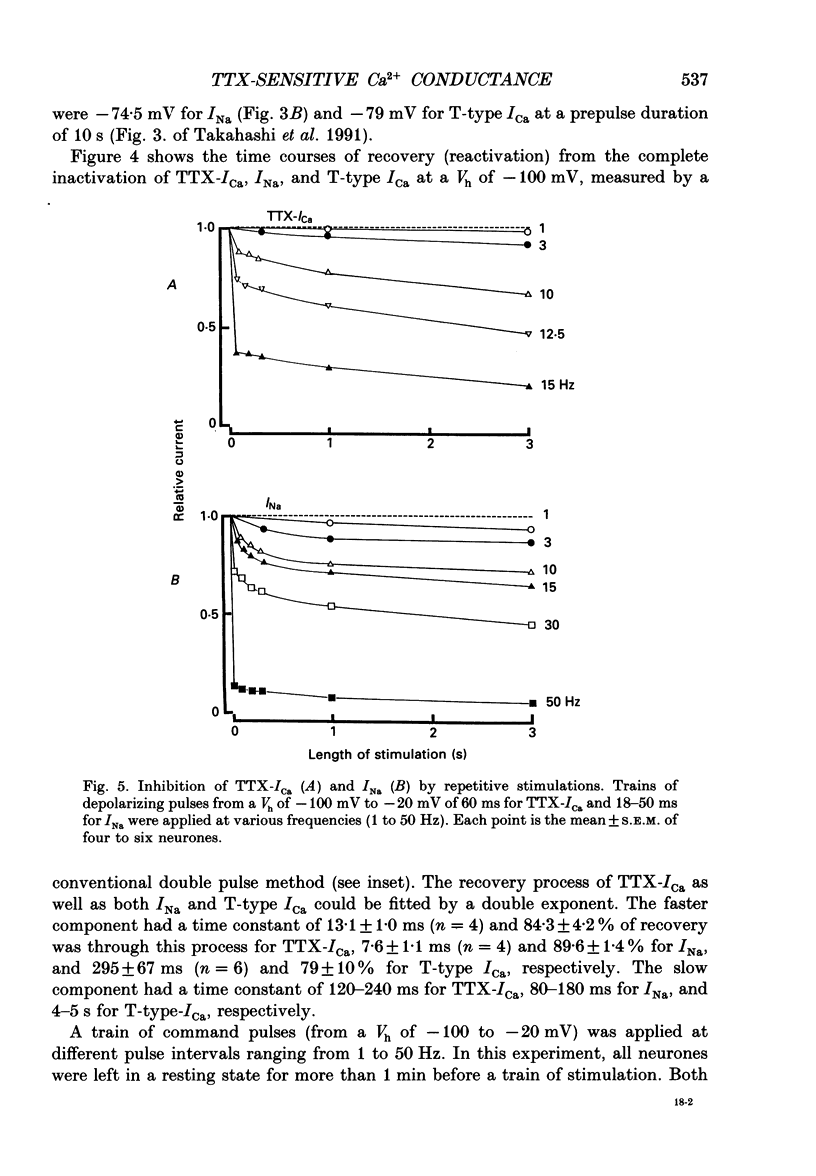
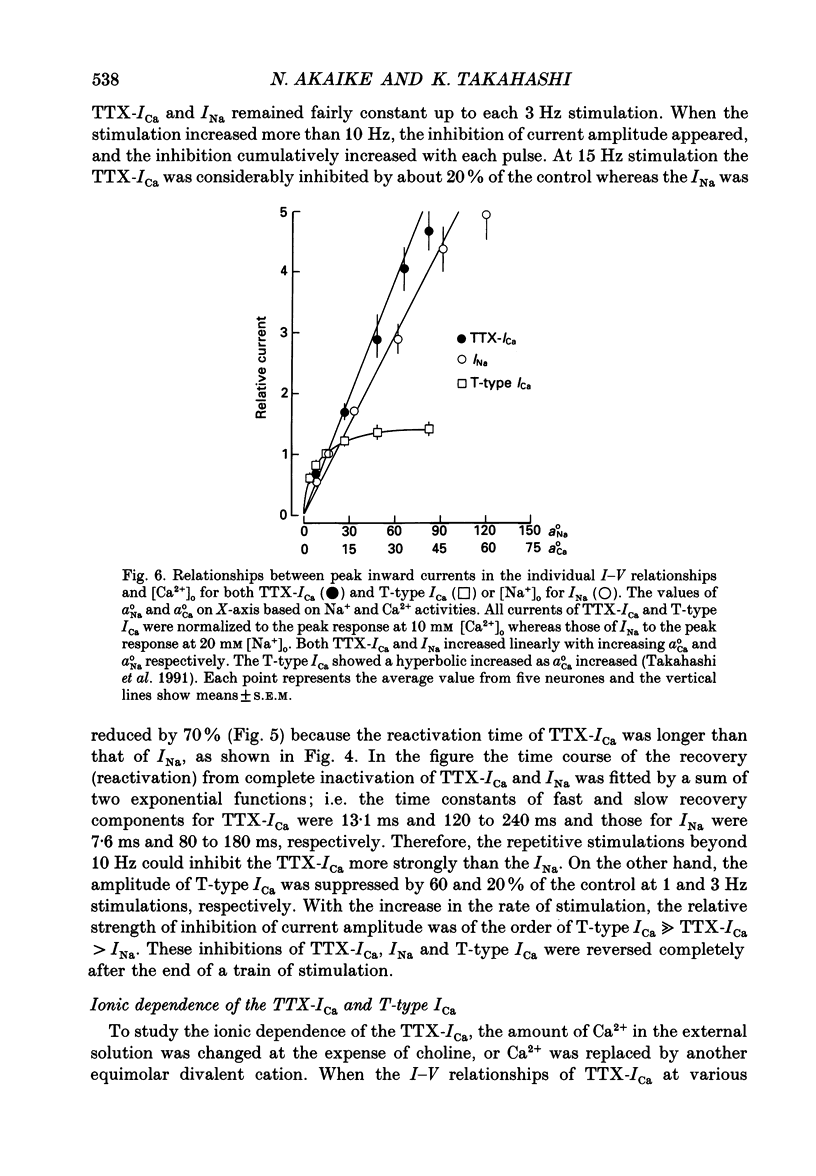
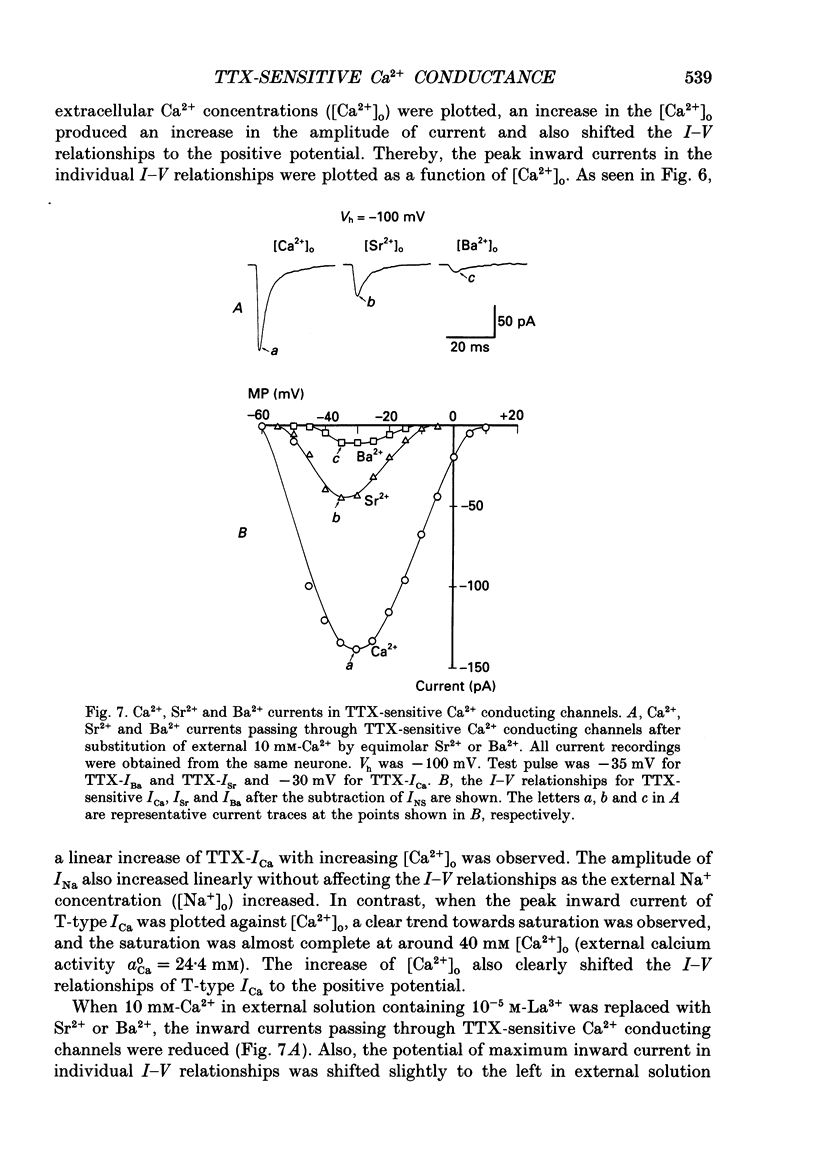
1. Tetrodotoxin (TTX)-sensitive Ca2+ conducting channels which produce a transient inward current were investigated in pyramidal neurones freshly dissociated from the dorsal part of rat hippocampal CA1 region by the use of the suction-pipette technique, which allows for intracellular perfusion under a single-electrode voltage clamp. 2. In all cells superfused with Na(+)- and K(+)-free external solution containing 10 mM-Ca2+ and 10(-5) M-La3+, a transient inward Ca2+ current was evoked by a step depolarization to potentials more positive than about -50 mV from a holding potential (VH) of -100 mV. This current was inhibited by either removing the extracellular Ca2+ or adding TTX (termed as 'TTX-ICa'). 3. Activation and inactivation processes of the TTX-ICa were highly potential dependent at 20-22 degrees C, and the latter was fitted by a double exponential function. The time to peak of the current decreased from 5.0 to 2.3 ms at a test potential change from -50 to 0 mV. The time constants of the current decay decreased from 2.8 to 2.2 ms for fast component (tau if) and from 16.0 to 8.2 ms for slow component (tau is) at a potential change from -35 to -10 mV. 4. The TTX-ICa was activated at threshold potential of about -55 mV and reached full activation at -30 mV. The steady-state inactivation of TTX-ICa could be fitted by a Boltzmann equation with a slope factor of 6.0 mV and a half-inactivation voltage of -72.5 mV. 5. Biphasic recovery (reactivation) from the complete inactivation of TTX-ICa was observed. The time constant of the major component (78.8 to 91.6% of total) of the reactivation was 13.1 ms, and that of the minor one was 120 to 240 ms. Therefore, TTX-ICa remained fairly constant at a train of stimulation up to 3 Hz. However, the inhibition of current amplitude occurred as the repetitive stimulation increased more than 10 Hz, and considerable tonic inhibition occurred with increasing stimulation frequency. 6. When the peak amplitudes in the individual current-voltage (I-V) relationships of TTX-ICa at various extracellular Ca2+ concentrations ([Ca2+]o) were plotted as a function of [Ca2+]o, the current amplitude increased linearly without showing any saturation. 7. The ratio of peak amplitude in the individual I-V relationships of Ca2+, Sr2+ and Ba2+ currents passing through the TTX-sensitive Ca2+ conducting channel was 1:0.33:0.05, although the current kinetics were much the same.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
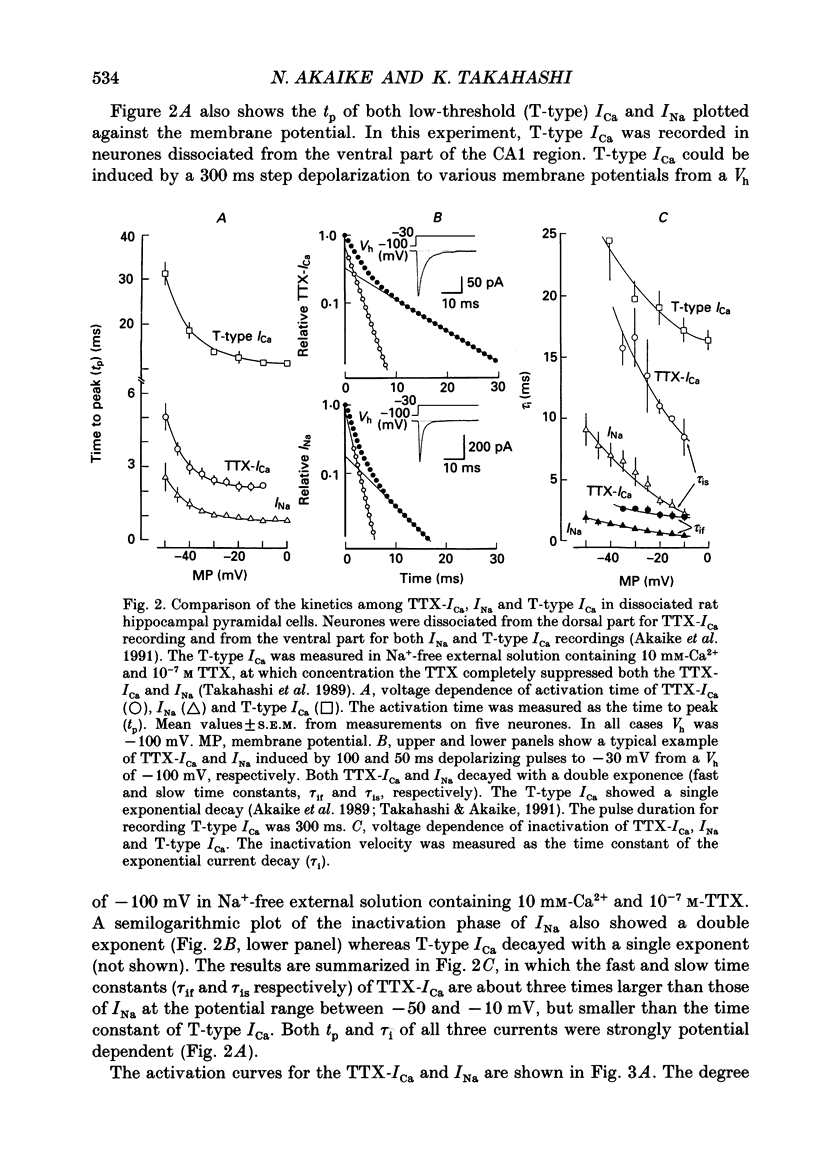
PDF


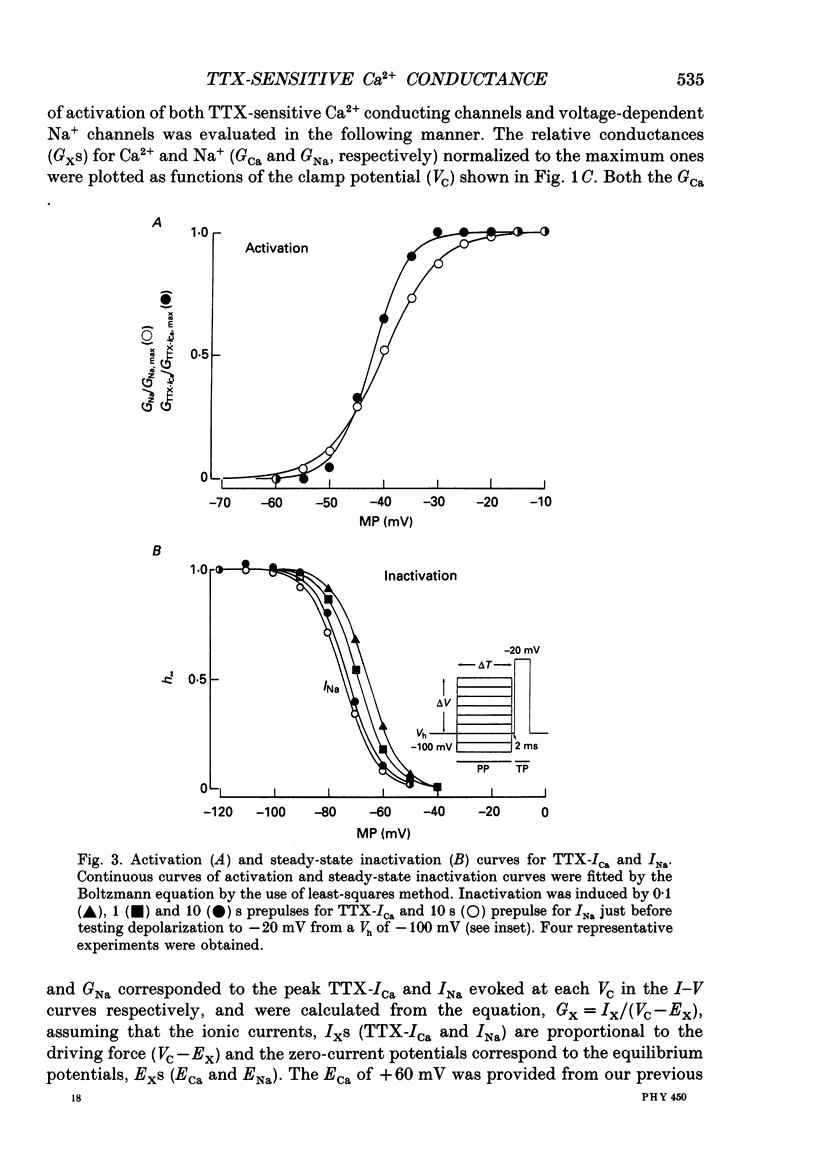
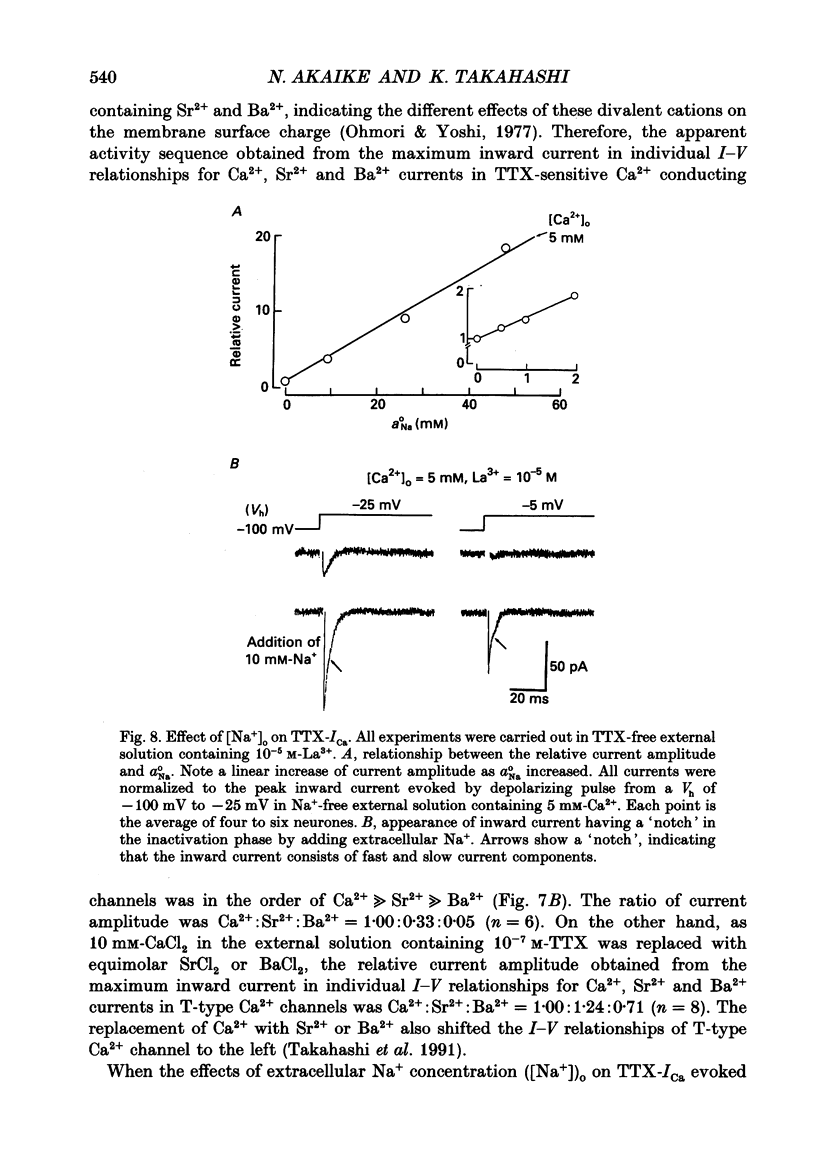

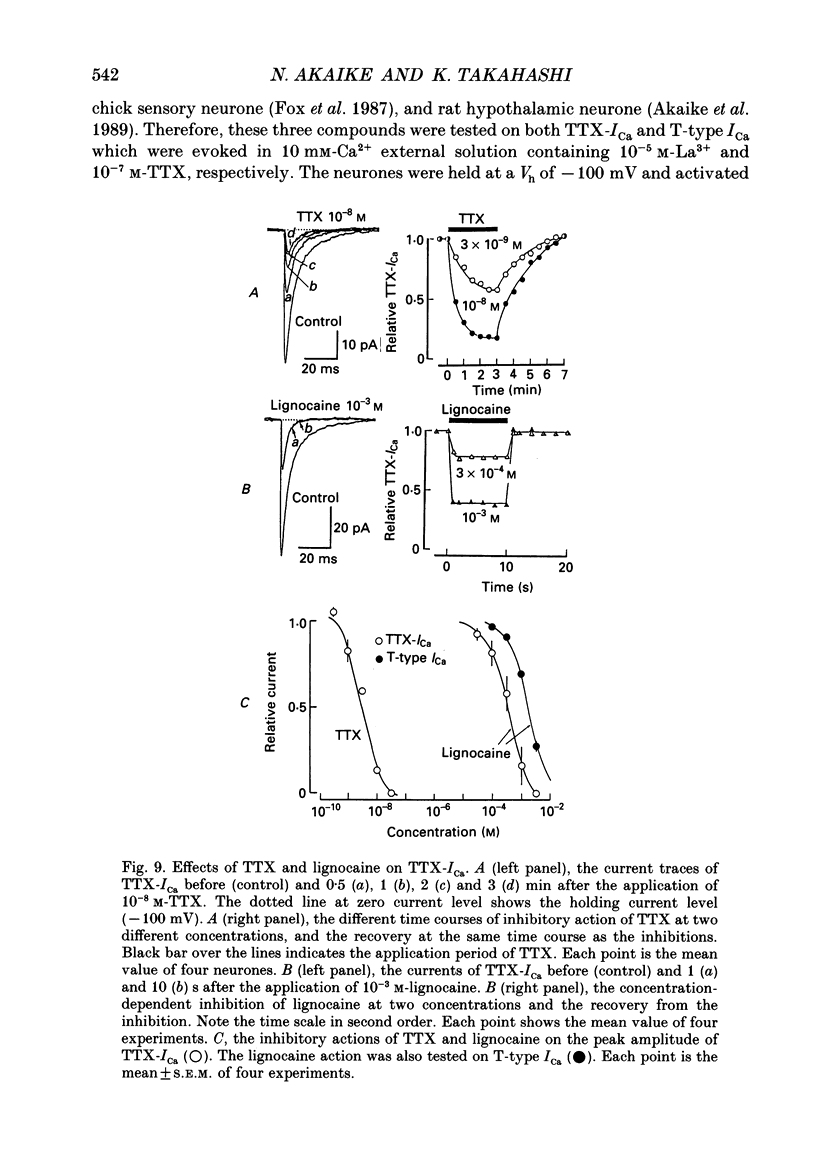
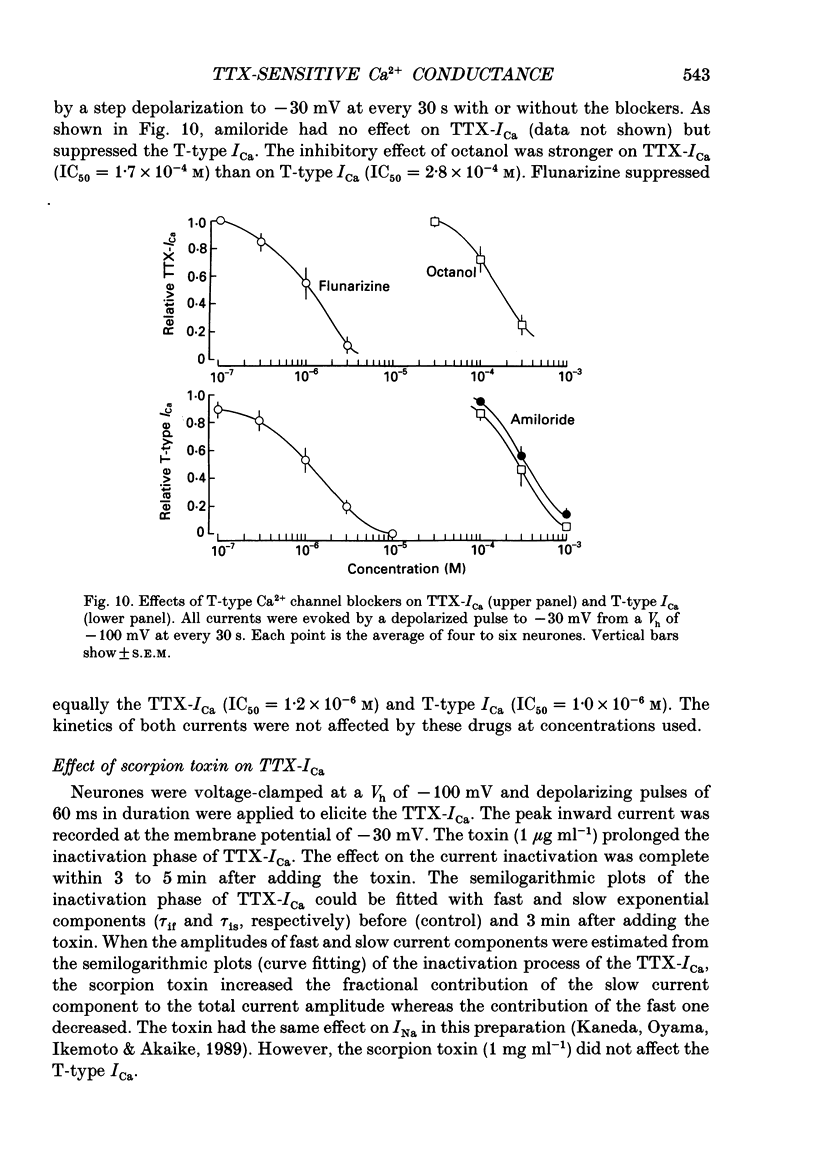



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Inoue M., Krishtal O. A. 'Concentration-clamp' study of gamma-aminobutyric-acid-induced chloride current kinetics in frog sensory neurones. J Physiol. 1986 Oct;379:171–185. doi: 10.1113/jphysiol.1986.sp016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Kostyuk P. G., Osipchuk Y. V. Dihydropyridine-sensitive low-threshold calcium channels in isolated rat hypothalamic neurones. J Physiol. 1989 May;412:181–195. doi: 10.1113/jphysiol.1989.sp017610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Takahashi K., Morimoto M. Heterogeneous distribution of tetrodotoxin-sensitive calcium-conducting channels in rat hippocampal CA1 neurons. Brain Res. 1991 Aug 9;556(1):135–138. doi: 10.1016/0006-8993(91)90557-c. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit E., Dubois J. M. Properties of maintained sodium current induced by a toxin from Androctonus scorpion in frog node of Ranvier. J Physiol. 1987 Feb;383:93–114. doi: 10.1113/jphysiol.1987.sp016398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. W. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988 Oct;11(10):465–469. doi: 10.1016/0166-2236(88)90200-7. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda M., Nakamura H., Akaike N. Mechanical and enzymatic isolation of mammalian CNS neurons. Neurosci Res. 1988 Apr;5(4):299–315. doi: 10.1016/0168-0102(88)90032-6. [DOI] [PubMed] [Google Scholar]
- Kaneda M., Oyama Y., Ikemoto Y., Akaike N. Scorpion toxin prolongs an inactivation phase of the voltage-dependent sodium current in rat isolated single hippocampal neurons. Brain Res. 1989 May 15;487(1):192–195. doi: 10.1016/0006-8993(89)90958-x. [DOI] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol. 1981 Jun;315:549–567. doi: 10.1113/jphysiol.1981.sp013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H., Vogel W. Calcium inward currents in internally perfused giant axons. J Physiol. 1973 Nov;235(1):225–265. doi: 10.1113/jphysiol.1973.sp010386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozhayeva G. N., Naumov A. P., Nosyreva E. D., Grishin E. V. Potential-dependent interaction of toxin from venom of the scorpion Buthus eupeus with sodium channels in myelinated fibre: voltage clamp experiments. Biochim Biophys Acta. 1980 Apr 24;597(3):587–602. doi: 10.1016/0005-2736(80)90230-8. [DOI] [PubMed] [Google Scholar]
- Narahashi T., Shapiro B. I., Deguchi T., Scuka M., Wang C. M. Effects of scorpion venom on squid axon membranes. Am J Physiol. 1972 Apr;222(4):850–857. doi: 10.1152/ajplegacy.1972.222.4.850. [DOI] [PubMed] [Google Scholar]
- Ohmori H., Yoshii M. Surface potential reflected in both gating and permeation mechanisms of sodium and calcium channels of the tunicate egg cell membrane. J Physiol. 1977 May;267(2):429–463. doi: 10.1113/jphysiol.1977.sp011821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Takahashi K., Yoshii M. Membrane currents of the tunicate egg under the voltage-clamp condition. J Physiol. 1976 Jan;254(3):607–638. doi: 10.1113/jphysiol.1976.sp011249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Takahashi K., Yoshii M. Two components of the calcium current in the egg cell membrane of the tunicate. J Physiol. 1976 Feb;255(2):527–561. doi: 10.1113/jphysiol.1976.sp011294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorbera L. A., Morad M. Atrionatriuretic peptide transforms cardiac sodium channels into calcium-conducting channels. Science. 1990 Feb 23;247(4945):969–973. doi: 10.1126/science.2154853. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Ueno S., Akaike N. Kinetic properties of T-type Ca2+ currents in isolated rat hippocampal CA1 pyramidal neurons. J Neurophysiol. 1991 Jan;65(1):148–155. doi: 10.1152/jn.1991.65.1.148. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Wakamori M., Akaike N. Hippocampal CA1 pyramidal cells of rats have four voltage-dependent calcium conductances. Neurosci Lett. 1989 Sep 25;104(1-2):229–234. doi: 10.1016/0304-3940(89)90359-5. [DOI] [PubMed] [Google Scholar]
- Tang C. M., Presser F., Morad M. Amiloride selectively blocks the low threshold (T) calcium channel. Science. 1988 Apr 8;240(4849):213–215. doi: 10.1126/science.2451291. [DOI] [PubMed] [Google Scholar]
- Tasaki I., Watanabe A., Lerman L. Role of divalent cations in excitation of squid giant axons. Am J Physiol. 1967 Dec;213(6):1465–1474. doi: 10.1152/ajplegacy.1967.213.6.1465. [DOI] [PubMed] [Google Scholar]
- Watanabe A., Tasaki I., Singer I., Lerman L. Effects of tetrodotoxin on excitability of squid giant axons in sodium-free media. Science. 1967 Jan 6;155(3758):95–97. doi: 10.1126/science.155.3758.95. [DOI] [PubMed] [Google Scholar]
- Yaari Y., Hamon B., Lux H. D. Development of two types of calcium channels in cultured mammalian hippocampal neurons. Science. 1987 Feb 6;235(4789):680–682. doi: 10.1126/science.2433765. [DOI] [PubMed] [Google Scholar]
- Yeh J. Z., Narahashi T. Kinetic analysis of pancuronium interaction with sodium channels in squid axon membranes. J Gen Physiol. 1977 Mar;69(3):293–323. doi: 10.1085/jgp.69.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]


