Abstract
In this report, we identify myogenin as an important transcriptional target under the control of three intracellular signaling pathways, namely, the p38 mitogen-activated protein kinase- (MAPK), calcium-calmodulin–dependent protein kinase- (CaMK), and calcineurin-mediated pathways, during skeletal muscle differentiation. Three cis-elements (i.e., the E box, myocyte enhancer factor [MEF] 2, and MEF3 sites) in the proximal myogenin promoter in response to these three pathways are defined. MyoD, MEF2s, and Six proteins, the trans-activators bound to these cis-elements, are shown to be activated by these signaling pathways. Our data support a model in which all three signaling pathways act in parallel but nonredundantly to control myogenin expression. Inhibition of any one pathway will result in abolished or reduced myogenin expression and subsequent phenotypic differentiation. In addition, we demonstrate that CaMK and calcineurin fail to activate MEF2s in Rhabdomyosarcoma-derived RD cells. For CaMK, we show its activation in response to differentiation signals and its effect on the cytoplasmic translocation of histone deacetylases 5 are not compromised in RD cells, suggesting histone deacetylases 5 cytoplasmic translocation is necessary but not sufficient, and additional signal is required in conjunction with CaMK to activate MEF2 proteins.
INTRODUCTION
Myogenic regulatory factors (MRFs) and myocyte enhancer factor (MEF) 2s are recognized as two families of transcription factors critically involved in skeletal muscle differentiation (Arnold and Winter, 1998; Lassar et al., 1994; Molkentin and Olson, 1996; Yun and Wold, 1996; Puri and Sartorelli, 2000). Among four members of MRFs, MyoD and Myf5 are involved in myogenic cell fate determination, whereas myogenin and MRF4 are involved in executing the differentiation program (Molkentin and Olson, 1996; Yun and Wold, 1996; Arnold and Winter, 1998). MEF2s can directly associate with MRFs to synergistically activate many muscle-specific genes (Molkentin et al., 1995; Molkentin and Olson, 1996).
In addition to MRFs and MEF2s, the homeodomain-containing Six proteins, the vertebrate homologs of the Drosophila sine oculis (so) product involved in Drosophila compound eye formation, also play important roles in myogenesis (Relaix and Buckingham, 1999; Kawakami et al., 2000). Genetic and biochemical analysis show that Six proteins physically associate with Eya, the vertebrate homologs of the Drosophila eye absent (eya) product, whereas Eya proteins directly bind Dach, the vertebrate homologs of the Drosophila Dachshund (dach) product (Pignoni et al., 1997; Heanue et al., 1999; Ohto et al., 1999). Among Six, Eya, and Dach proteins, only Six proteins are known to directly bind DNA (e.g., the MEF3 site in the myogenin promoter) (Spitz et al., 1998). However, both Six/Eya and Eya/Dach complexes are shown to synergistically induce myogenesis and MEF3-containing genes (Heanue et al., 1999; Ohto et al., 1999).
Although the biological roles of MRFs, MEF2s, and Six proteins in myogenesis are well established, the question of how these transcription factors are in turn regulated by intracellular signaling pathways is poorly understood. Recently, we showed the insulin-like growth factors (IGFs)/phosphatidylinositol 3-kinase (PI3K)/Akt-mediated pathway increases myogenin transcription by activating both MyoD and MEF2 proteins, which then bind the specific sites in the promoter to induce myogenin expression (Florini et al., 1991; Coolican et al., 1997; Tamir and Bengal, 2000; Xu and Wu, 2000). We and others also showed that the p38 mitogen-activated protein kinase (MAPK) enhances the transcriptional activity of both MyoD and MEF2C/2A (Zetser et al., 1999; Zhao et al., 1999; Han et al., 1997; Wu et al., 2000b). Although our data suggest the p38 MAPK acts early during differentiation, the immediate downstream myogenic genes under the control of MyoD, MEF2s, and the p38 MAPK pathway have not been well characterized.
In addition, calcineurin, a calcium-calmodulin–activated serine/threonine phosphatase, is found to activate MEF2s in several different cell types (Blaeser et al., 2000; Youn et al., 2000). In skeletal muscles, activation of MEF2s by calcineurin mediates electrical signals from motor neurons to determine the muscle fiber types (Wu et al., 2000a). However, this process mainly occurs in innervated, mature muscle fibers. Whether the calcineurin-mediated pathway plays any role during early muscle differentiation is not convincingly addressed. Two recent reports suggest that calcineurin can enhance early myogenic differentiation either by increasing myogenin expression (Friday et al., 2000) or by stimulating MyoD-mediated myogenic conversion of 10T1/2 fibroblasts (Delling et al., 2000). However, the detailed mechanism by which calcineurin regulates myogenin expression in the former case and the identity of the key early myogenic genes under the control of calcineurin in the latter remain unclear.
Recently, CaMKIV, a calcium-calmodulin–dependent protein kinase, was also shown to activate MEF2s by dissociating class II histone deacetylases (e.g., HDAC5) from MEF2s, thus relieving the transcriptional repressive effect of HDACs (Lu et al., 2000a,b; McKinsey et al., 2000a,b). However, in myogenic cells, the identity of the key early myogenic genes controlled by MEF2s and the CaMK-mediated pathway remains unknown.
In this study, we demonstrate that myogenin is a key early myogenic gene under the influence of the p38 MAPK, CaMK-, and calcineurin-mediated signaling pathways. These signaling pathways significantly enhance myogenin transcription. Several key cis-elements in the myogenin promoter in response to these pathways are defined. MyoD, MEF2, and Six proteins that bind these cis-elements are shown to be activated by these three signaling pathways. The relationship among the three pathways is studied and the ability of the constitutively active CaMK and calcineurin to activate MyoD and MEF2s in Rhabdomyosarcoma-derived RD cells is also investigated.
MATERIALS AND METHODS
Cell Lines and Antibodies
C2C12 (American Type Culture Collection, Manassas, VA) and 10T1/2MyoD cells were cultured in DMEM supplemented with 20% fetal bovine serum and antibiotics (growth medium, or GM). To induce differentiation, near confluent C2C12 cells were grown in DMEM supplemented with 2% horse serum (differentiation medium, or DM). HeLa, 10T1/2, and RD cells were grown in DMEM plus 10% fetal bovine serum. Antibodies used in this work included anti-sarcomeric myosin heavy chain (MF20), anti-myogenin (F5D), anti-β-actin, and anti-hemagglutinin (HA). Except for MF20 (Developmental Studies hybridoma bank), all other antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA).
Plasmids
Various forms of CaMKIV, CaMKK, calcineurin, 4RE-luc, 3MEF2-luc, gal4-luc, gal4MyoD, gal4MEF2 (full length), HA-p38α, GST-ATF2 (1-92), HA-MKK6EE, and HDAC5-GFP were described previously (Enslen et al., 1996; Werlen et al., 1998; Wu et al., 2000b). Construction of the mouse myogenin promoter G133-luc and its derivatives [G133E, G133MEF2, G133MEF3, G133(E+2), G133(E+3), G133(2+3), and G133TM] were also described previously (Xu and Wu, 2000). The following primers were used to generate G133(E+2+I) and G133(E+3+I): forward, P83: 5′ TGT GCA GCA ACA taT gAG AGG GGG GCT C 3′; and reverse, P84: 5′ GAG CCC CCC TCT cAt aTG TTG CTG CAC A 3′ (lowercase letters indicate where mutations are introduced). 3XMEF3-Luc reporter plasmid was constructed by inserting the oligo pair 5′ (forward) CCG GCT CAG GTT TCC TTT TCA GGT TTC CTT TTC AGG TT and 5′ (reverse) TCG AAA CCT GAA AAG GAA ACC TGA AAA GGA AAC CTG AG upstream of the thymidine kinase core promoter.
Northern Blot
Total RNA was extracted from 1 × 107 cells with TRIzol reagent (Invitrogen, Carlsbad, CA) following manufacturer's suggestions. Total RNA (20 μg) was resolved in a 1% agarose gel, transferred and cross-linked (using a UV cross-linker; Stratagene, La Jolla, CA) to a Hybond-N+ membrane (Amersham Biosciences, Piscataway, NJ), and hybridized with a 32P-labeled probe derived from cDNA encoding either mouse myogenin or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) essentially as described previously (Xu and Wu, 2000).
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted as described above. Total RNA (500 ng) was subjected to a one-step RT-PCR using Access RT-PCR kit (Promega, Madison, WI). Primer pairs used were as follows: myogenin (forward: 5′ GAC TCC CCA CTC CCC ATT CAC ATA; reverse: 5′ GGC GGC AGC TTT ACA AAC AAC ACA) and GAPDH (forward: 5′ TGA TGC TGG TGC TGA GTA TGT CGTG; reverse: 5′ TCC TTG GAG GCC ATG TAG GCC AT).
Transfection and Reporter Assays
Cells were first transfected with various DNA using LipofectAMINE Plus kit (Invitrogen) and cultured in GM for 36 h. Cells were than cultured in DM for 24 h followed by cell lysis for luciferase activity determination. Whole cell extracts (WCEs) were prepared by lysing cells in lysis buffers (50 mM HEPES, pH 7.6, 1% Triton X-100, 150 mM NaCl, 1 mM EGTA, 1.5 mM MgCl2, 100 mM NaF, 20 mM p-nitrophenylphosphate, 20 mM β-glycerolphosphate, 50 μM sodium vanadate, 2 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 2 μg/ml aprotinin, 0.5 μg/ml leupeptin, and 0.7 μg/ml pepstatin) followed by removal of insoluble debris with a tabletop minicentrifuge (12,000 rpm/2 min). Luciferase activity was determined with a luciferase assay kit (Roche Applied Science, Indianapolis, IN) and a Monolight 2010 luminometer (Analytical Luminescence Laboratory, San Diego, CA). Luciferase units were normalized against total protein amount present in each sample.
Drug Treatment
Pathway-specific drugs were added to the culture media at the time of media change from GM to DM. The final concentration of drugs used was as follows: 10 μM SB202190, 25 μM LY294002, 10 μM KN93, 20 μM KN62, 15 μM cyclosporin A (CsA), 100 μM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid/acetyloxymethyl ester (BAPTA/AM), 10 μM nifedipine, and 25 μM PD98059. WCE or total RNA was prepared 10 h after the drug treatment and subsequently subjected to various analyses (i.e., Western blot, reporter assays, Northern blot, and RT-PCR).
Immunostaining and Immunofluorescence Imaging
At times indicated in the figure legends, cells (grown on coverslips) were fixed for 15 min in 4% paraformaldehyde, permeabilized with 0.2% Triton X-100 in phosphate-buffered saline for 15 min at room temperature, and stained with specific antibodies as indicated. The coverslips were then mounted on slides with a few drops of Mowiol (Calbiochem, San Diego, CA). The fluorescent images were obtained using a Nikon immunofluorescence microscope (Optiphot-2) linked to a charge-coupled device camera.
Kinase Assays
His or HA-tagged kinases from 50 or 100 μg of WCE were pulled down with either Talon beads (CLONTECH, Palo Alto, CA) or specific antibodies and protein A-Sepharose beads, respectively, and washed. To measure the kinase activity of His-CaMKIV, the pulled-down kinases were incubated with 40 μM syntide-2 in the kinase buffer (20 mM HEPES, pH 7.6, 10 mM MgCl2, 20 mM β-glycerolphosphate, 20 μM ATP, 1 mM CaCl2, 10 μM calmodulin, and 10 μCi of [γ-32P]ATP). The reactions were carried out at 30°C for 30 min, terminated with trichloroacetic acid, subjected to the standard P-81 filter paper assay, and 32P incorporation was determined with a scintillation counter. For immunoprecipitated HA-p38 MAPK, 1 μg of GST-ATF2 (1-92) was added to the same kinase buffer as described above (except without CaCl2 and calmodulin), and reactions were incubated at 30°C for 30 min. The kinase mixtures were resolved by SDS-PAGE, and labeled protein bands were visualized by autoradiography.
Western Blot
Cells were harvested in the above-described lysis buffer and 30 μg of WCE was resolved by SDS-PAGE, transferred to a membrane (Immobilon-P; Millipore, Bedford, MA), and probed with various antibodies. Protein bands were visualized using ECL kit (Amersham Biosciences).
RESULTS
Myogenin Is Under Transcriptional Control of p38 MAPK-, CaMK-, and Calcineurin-mediated Signaling Pathways
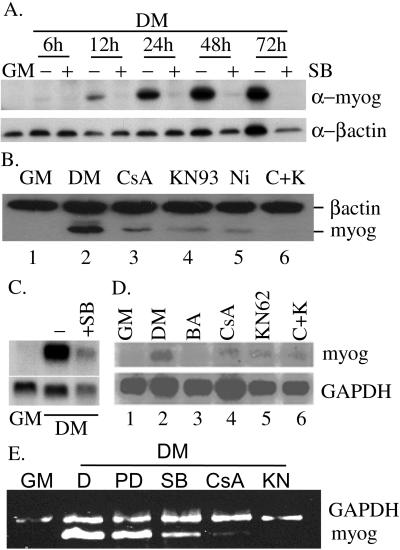
In previous studies by us and others, it was known that specific inhibition of the p38 MAPK activity with the inhibitor SB202190 blocks muscle cell differentiation and myogenin protein expression (Cuenda and Cohen, 1999; Zetser et al., 1999; Wu et al., 2000b). In addition, we and others also found that KN93 and CsA, drugs specifically inhibiting CaMKs and calcineurin, respectively, also partially inhibited myogenin expression (Figure 1B; Friday et al., 2000). These data suggested that myogenin expression might be under the control of the p38 MAPK-, CaMK-, and calcineurin-mediated pathways. To fully understand the molecular mechanisms underlying the regulation of myogenin by various intracellular signaling pathways, we first examined the myogenin protein expression profile during differentiation of mouse C2C12 cells in either the absence or presence of various pharmacological inhibitors. SB202190, KN93, CsA, and nifedipine are specific inhibitors for the p38 MAPK, CaMK, calcineurin, and the L-type membrane calcium channels, respectively. As shown in Figure 1A, expression of myogenin was almost completely blocked by 10 μM SB202190 as early as 12 h after cells were grown in DM, which was the time when myogenin just started to be expressed in this in vitro differentiation system. Myogenin protein expression was also significantly reduced by KN93, CsA, and nifedipine (Figure 1B). Addition of KN93 and CsA together further reduced the myogenin expression (Figure 1B, compare lane 6 with lanes 3 and 4), suggesting the CaMK and calcineurin may act cooperatively on two separate pathways. The fact that nifedipine could also block myogenin expression suggested that it was the extracellular calcium influx through the L-type membrane Ca2+ channels that was mainly responsible for subsequent activation of CaMK and calcineurin.
Figure 1.
Inhibition of the p38 MAPK-, CaMK-, and calcineurin-mediated signaling pathways blocks myogenin expression. (A) When C2C12 cells were near confluent, the media were changed from GM to DM in the absence or presence of 10 μM SB202190. WCEs were made at indicated time points and subjected to Western blot analysis. (B) Various drugs were added to near confluent C2C12 cells at the time of media change from GM to DM. Ten hours after drug treatment, WCE were prepared and subjected to Western blot analysis. Dimethyl sulfoxide was added to the sample without drug treatment. The final concentration of drugs used was as follows: 15 μM CsA, 10 μM KN93, and 10 μM nifedipine (Ni). C+K, both CsA and KN93 were added. (C and D) Total RNA was extracted, and 20 μg of total RNA was subjected to Northern blot analysis. (E) Total RNA (500 ng) was subjected to RT-PCR by using primers specific for mouse myogenin and GAPDH. Polymerase chain reaction products were separated and visualized on a 2% agarose gel. The drug treatment scheme in C and D and E was similar to that in B, except KN62 (20 μM), PD98059 (PD, 25 μM), and BAPTA/AM (BA, 100 μM final) were also used. (D) Dimethyl sulfoxide. All experiments described above were done twice with similar results. A representative picture for each experiment is shown herein.
To understand the nature of myogenin regulation by these three pathways, we also examined myogenin mRNA level in either the absence or presence of various drugs in a Northern blot assay. Indeed, myogenin mRNA level was drastically reduced in the presence of SB202190 (Figure 1C) and partially reduced by KN62, CsA, and BAPTA/AM (a membrane-permeable specific chelator for intracellular calcium) (Figure 1D). Similar result was also obtained using RT-PCR (Figure 1E). This suggested that the p38 MAPK-, CaMK-, and calcineurin-mediated signaling pathways regulate myogenin expression at the mRNA level. The stronger reduction in myogenin mRNA level by BAPTA/AM treatment confirmed a key role for calcium in myogenin regulation (Figure 1D, lane 3).
Deliberate Activation of p38 MAPK-, CaMK-, and Calcineurin-mediated Pathways Increases Endogenous Myogenin Expression and Enhances Muscle Differentiation
Because the above-mentioned loss-of-function data generated with pharmacological inhibitors could potentially be due to nonspecific effects of the drugs, we also tested whether deliberate activation of any one of the pathways described could enhance the expression of myogenin and phenotypic differentiation. MKK6EE, CaMKIVc, and calcineurin AΔ (CnAΔ) are constitutively active signaling molecules used in our subsequent assays (Enslen et al., 1996; Werlen et al., 1998; Wu et al., 2000b). We transiently transfected C2C12 cells with either an empty expression vector or vectors encoding one of the constitutively active signaling molecules and then let cells differentiate in DM for 24 h. We then examined the cell morphology by microscopy and the endogenous myogenin protein expression by immunoblot. As shown in Figure 2A, the cells transfected with either the constitutively active MKK6EE, CaMKIVc, or CnAΔ showed enhanced and accelerated differentiation as manifested by increased appearance of the muscle-specific myosin heavy chain. In addition, an increase in the expression of endogenous myogenin was also seen in cells transfected with plasmids encoding either MKK6EE, CaMKIVc, or CnAΔ compared with cells transfected with the empty vector (Figure 2B). This result indicated that signals initiated from these constitutively active signaling molecules could indeed target the endogenous myogenin promoter. When individual transfected cells were examined for myogenin expression using immunostaining technique, indeed cells transfected with the constitutively active molecules (green) showed enhanced myogenin staining (red) (Figure 2C).
Figure 2.
Deliberate activation of the p38 MAPK-, CaMK-, and calcineurin-mediated pathways enhances myogenic differentiation and endogenous myogenin expression. C2C12 cells were transfected with various plasmids as indicated. After 36 h of growth in GM and 24 h of growth in DM, cells were either fixed and stained with various antibodies/or 4,6-diamidino-2-phenylindole (DAPI) (A and C) or lysed to prepare WCE (B). (A) Cells were stained with anti-MHC (top) or DAPI (bottom). Vec, empty expression vector. (B) WCEs were subjected to Western blot analysis. (C) C2C12 cells were cotransfected with a plasmid encoding GFP along with either an empty vector or one of the three constitutively active molecules as indicated. Cells were fixed after 12 h of growth in DM, stained with anti-myogenin antibody, and subsequently visualized for GFP (top) and myogenin (bottom). Please note that the cells stained positive for myogenin in the (empty) vector-transfected cells (the leftmost column) were not the same as those positive for GFP.
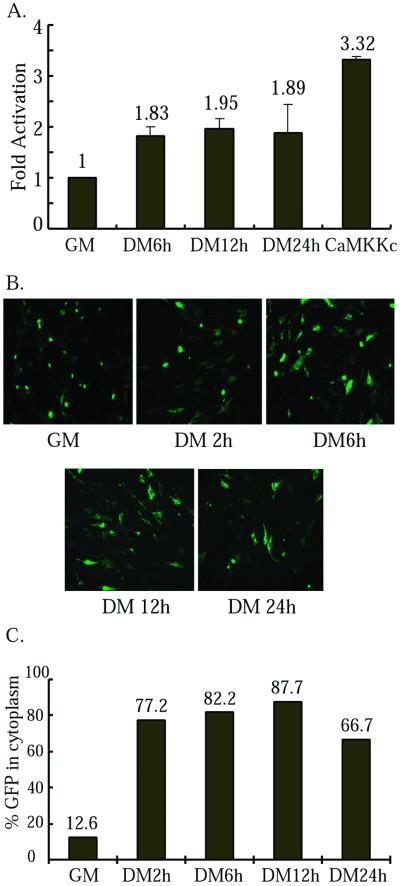
We and others showed previously that the p38 MAPK kinase activity and the calcineurin phosphatase activity increased upon muscle differentiation (Delling et al., 2000; Wu et al., 2000b). No one has directly looked at the CaMK activity before and after differentiation. To address this issue, we measured the kinase activity of a transfected CaMK during differentiation. As shown in Figure 3A, the CaMK activity slightly but consistently increased upon differentiation. Because the CaMK activity was linked to HDAC5 cytoplasmic shuttling (McKinsey et al., 2000a,b), we also examined cytoplasmic translocation of a HDAC5 fused with green fluorescent protein (HDAC5-GFP) during C2C12 differentiation. As early as 2 h after cells were placed in DM, a significant portion of HDAC5-GFP (77.2 vs. 12.6% in GM) was already present in the cytoplasm (Figure 3, B and C). This clearly indicates activation of the CaMK well precedes the expression of myogenin (which normally appears after 10 h in DM). The data given above indicated that the p38 MAPK-, CaMK-, and calcineurin-mediated pathways play positive and decisive roles in early muscle differentiation by participating in regulation of myogenin expression.
Figure 3.
CaMKIV kinase activity increases during C2C12 differentiation. (A) C2C12 cells were cotransfected with His-CaMKIV along with either an empty vector or a constitutively active CaMKK (positive control), and cells were harvested at times indicated (CaMKK, DM 24 h). After normalization of CaMKIV level by Western blot, the kinase activity of the CaMKIV toward syntide in each sample was determined. Fold activation: the ratio of the kinase activity in DM over that in GM. The experiment was done twice and the mean ± SD is shown. A two-sample t test was performed with p < 0.05 considered statistically significant. (B) C2C12 cells were transfected with HDAC5-GFP. Cells were fixed at times indicated and visualized under fluorescent microscope. (C) The average ratio of cells containing HDAC5-GFP in the cytoplasm over those with green colors (i.e., total transfected cells) from five random fields in each sample from B is plotted.
Myogenin Promoter Regulation by p38 MAPK-, CaMK-, and Calcineurin-mediated Pathways Requires E box, MEF2, and MEF3 Sites
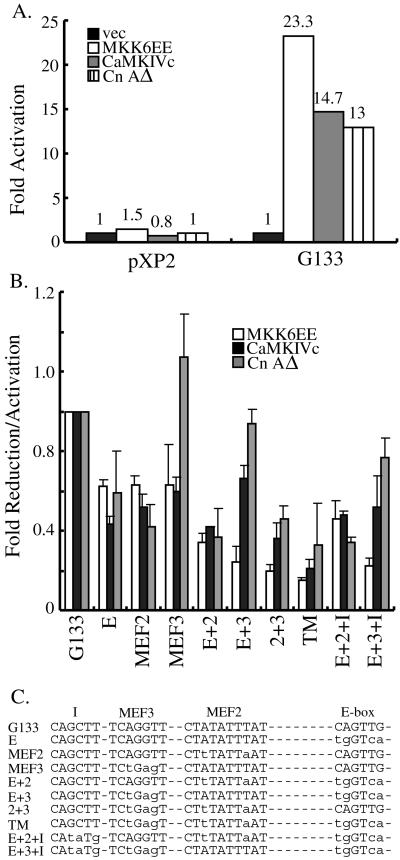
To further prove that the p38 MAPK-, CaMK-, and calcineurin-mediated pathways increase myogenin gene transcription, we used a reporter assay using a 133-base pair proximal myogenin promoter linked to a luciferase reporter gene. This short fragment of myogenin promoter was shown to contain sufficient regulatory elements for somite-restricted expression of a lacZ transgene and for specific response to the IGF/PI3K/Akt signaling pathway (Yee and Rigby, 1993; Xu and Wu, 2000). When deliberately activated, all three pathways could activate the promoter with MKK6EE being the strongest stimulus (Figure 4A). This activation of myogenin promoter was also specific, because the same luciferase construct (pXP2) without the 133-base pair myogenin promoter failed to be activated by either MKK6EE, CaMKIVc, or CnAΔ (Figure 4A).
Figure 4.
Mapping of the cis-elements in myogenin promoter responsive to the p38 MAPK-, CaMK-, and calcineurin-mediated pathways. (A) Luciferase constructs without (pXP2) or with the 133-base pair proximal myogenin promoter (G133-luc) were separately transfected into C2C12 cells along with either an empty expression vector (vec) or expression vectors encoding constitutively active signaling molecules. The transfection scheme was the same as described in the Figure 2 legend. Luciferase activity was measured and normalized against total protein present in the extracts. Fold activation was the ratio of luciferase activity of samples transfected with the active signaling molecules over that transfected with an empty vector. The experiment was repeated five times, and a representative set of values is presented. (B) C2C12 cells were transfected with either G133-luc or various mutant myogenin pro moter reporters along with different constitutively active signaling molecules as indicated. Fold reduction/activation was the ratio of the luciferase activity of various mutant myogenin reporters over that of G133-luc. The experiment was repeated five times and the mean ± SD was presented. When each mutant was compared with the wild type, a two-sample t test was performed with p < 0.05 considered statistically significant. The symbols underneath the bar graph indicated in which cis-element mutations were introduced in the G133-luc. TM, the triple mutant in which the E box, MEF2, and MEF3 sites were all mutated as shown in C. (C) Schematic showing the key cis-elements mutated in the 133-base pair proximal myogenin promoter. Lowercase letters indicate where mutations were introduced. Site I is a putative cis-element conserved in the mouse, chicken, and human proximal myogenin promoters.
We next sought to identify the cis-elements in the myogenin promoter responsive to each of the three signaling pathways. Recently, we showed that a unique E box, MEF2, and MEF3 sites in the mouse proximal 133-base pair myogenin promoter were critical cis-elements responsive to the IGF/PI3K/Akt signaling pathway (Xu and Wu, 2000). To test whether these sites were also involved in activation of the myogenin promoter by these three signaling pathways, we transfected myogenic C2C12 cells with various combinations of plasmids encoding the constitutively active signaling molecules and luciferase reporters under the control of different mutant myogenin promoters. As shown in Figure 4B, all three myogenin reporters with single-site mutation displayed reduced activities in response to either the constitutively active MKK6EE or CaMKIVc. Interestingly, only G133E and G133MEF2 showed reduced activities in response to CnAΔ, whereas the responsiveness of G133MEF3 to CnAΔ was as good as that of the wild-type G133, suggesting the MEF3 site was not absolutely required for calcineurin to activate the myogenin promoter. Because significant activation of the single-site mutant promoters could still be seen, it suggested multiple cis-elements were responsive to the activating kinases and phosphatase (Figure 4B). We then tested the responsiveness of the promoters harboring mutations at two of the three critical cis-elements. A further decrease in activities was observed in most of the reporters with double-site mutation compared with their single-site mutation counterparts with the exception of G133(E+3) in response to the constitutively active CaMK and calcineurin (Figure 4B). It was interesting to note that G133(E+3) (in which the MEF2 site remained intact) could still be significantly activated by CaMK and calcineurin compared with the other two reporters with double-site mutation. This suggested that activation of the myogenin promoter by CaMK and calcineurin is mainly mediated by the MEF2 site. The promoter with triple mutations (G133TM) in which the E box, MEF2, and MEF3 sites were all mutated had further decreased responsiveness to MKK6EE and CaMKIVc, suggesting all three sites responded to the p38 MAPK- and CaMK-mediated signals. In contrast, the responsiveness of G133TM to calcineurin was similar to that of G133(E+2), in agreement with the previous notion that the MEF3 site in the myogenin promoter did not respond well to the calcineurin-mediated signal.
To prove that the three cis-elements identified above were specific, we mutated another conserved region (site I) in G133(E+2) and G133(E+3) (Figure 4C). Like the E box, and MEF2 and MEF3 sites, this site I (CAGCTTAG) was completely conserved in the chick, mouse, and human 133-base pair proximal myogenin promoters (Xu and Wu, 2000). Unlike G133TM, however, when the site I was mutated in the G133(E+2) or G133(E+3) background [i.e., G133(E+2+I), G133(E+3+I)], no significant further decrease in their responsiveness to the three pathways was detected compared with that of their double-site mutation counterparts (Figure 4B). This indicated that the three cis-elements (i.e., E box, MEF2, and MEF3 sites) are specific response elements to the p38 MAPK-, CaMK-, or calcineurin-mediated pathways.
MyoD, MEF2s, and Six Proteins Are Common Nuclear Targets for p38 MAPK-, CaMK-, and Calcineurin-mediated Signaling Pathways
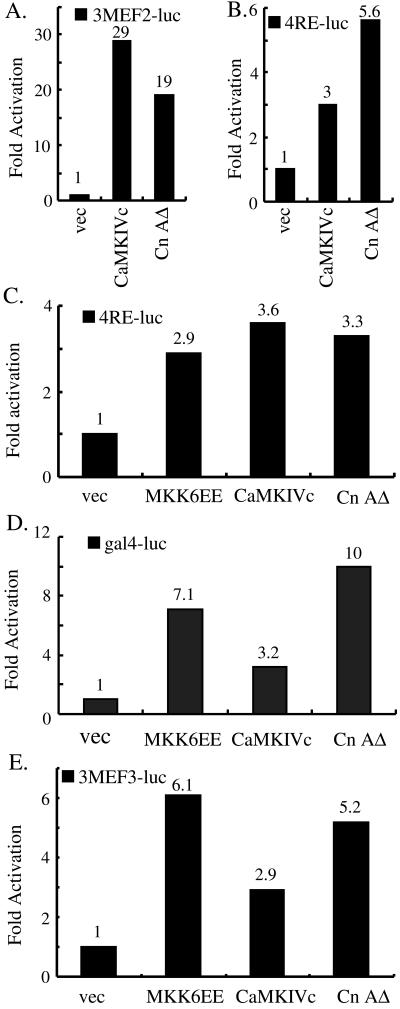
The implication of the E box, MEF2, and MEF3 sites in the myogenin promoter in response to the p38 MAPK-, CaMK-, and calcineurin-mediated signaling pathways suggested that MyoD, MEF2, and Six proteins, which bind the above-mentioned sites, respectively, are the nuclear signal receivers mediating the stimulatory effect of the signaling pathways. Indeed, we and others demonstrated previously that 38 MAPK could not only directly phosphorylate and activate MEF2A and 2C but also enhance transcriptional activity of MyoD (Han et al., 1997; Zetser et al., 1999; Zhao et al., 1999; Wu et al., 2000b). For the CaMK- and calcineurin-mediated pathways, we also consistently observed the activation of either MEF2-dependent (3MEF2-luc) or MyoD-dependent (4RE-luc) reporters by the constitutively active CaMK and calcineurin in C2C12 cells (Figure 5, A and B). The stimulatory effect of CaMK and calcineurin on MEF2s was more pronounced than that on MyoD. The strong stimulatory effect of CaMK and calcineurin on MEF2 was in agreement with several recent reports (Blaeser et al., 2000; Lu et al., 2000a,b; Wu et al., 2000a; Youn et al., 2000), however, the effect of CaMK and calcineurin on MyoD was revealed for the first time. To further prove that MyoD could indeed serve as a target for CaMK and calcineurin, we transfected 4RE-luc and the constitutively active signaling molecules into 10T1/2 cells stably expressing MyoD (10T1/2MyoD cells). MKK6EE was used as a positive control (Puri et al., 2000; Wu et al., 2000b). In agreement with the data from C2C12 cells, MyoD could also be activated in 10T1/2 cells by CaMK and calcineurin (Figure 5C).
Figure 5.
Transcriptional activity of MyoD and MEF2 is activated by the CaMK- and calcineurin-mediated signaling pathways. (A and B) C2C12 cells were transfected with either 3MEF2-luc (A) or 4RE-luc (B) in combination with either an empty vector, the constitutively active CaMKIVc, or CnAΔ. (C) 10T1/2 cells stably expressing MyoD were transfected with 4RE-luc along with various plasmids as indicated. (D) HeLa cells were transfected with gal4-luc, gal4MyoD along with either an empty expression vector or various active signaling molecules as indicated. (E) C2C12 cells were transfected with 3MEF3-luc in combination with vectors encoding signaling molecules as indicated. WCE were made for luciferase activity determination either 24 h (for assays in A, B, D, and E) or 48 h (for assays in C) after cells were grown in DM. All of the above-mentioned experiments were repeated three times, and a representative is shown. The numbers on top of each bar indicate the fold activation.
Next, we asked whether the activation of MyoD by CaMK and calcineurin occurred independently of its DNA binding activity. We fused a full-length MyoD with the yeast gal4 DNA binding domain (gal4MyoD) and cotransfected HeLa cells with the fusion gene and a gal4-dependent reporter (gal4-luc) in combination with various plasmids as indicated in Figure 5D. Indeed, like MKK6EE (positive control), CaMKIVc and CnAΔ could activate MyoD by enhancing its transcriptional activity, because any effect on the DNA binding ability of MyoD is less likely to be revealed in this assay system. To directly examine the effect of the active signaling molecules on the DNA binding ability of MyoD, a gel mobility shift assay was carried out using the same cell extracts of 10T1/2MyoD as used in Figure 5C and the 32P-labeled E box as a probe. No obvious change in the DNA binding activity of MyoD could be detected (data not shown). In agreement with the reporter assay described above, the results shown herein strongly suggest that MyoD is another important downstream target of CaMK and calcineurin in addition to MEF2s and that CaMK and calcineurin activate MyoD by enhancing its transcriptional activity.
Because Six1 and 4 are present in C2C12 cells and could bind the MEF3 site in the myogenin promoter and activate a luciferase reporter under the control of multimerized MEF3 sites (Spitz et al., 1998), we tested whether the constitutively active signaling molecules could activate a MEF3-containing reporter (3MEF3-luc) in C2C12 cells. As expected, MKK6EE, CaMKIVc, and CnAΔ all activated the MEF3 reporter (Figure 5E). As a negative control, a constitutively active JNKK2CA that activates the c-Jun NH2-terminal kinase pathway failed to activate this reporter (our unpublished data).
Cross Talk among Different Signaling Pathways
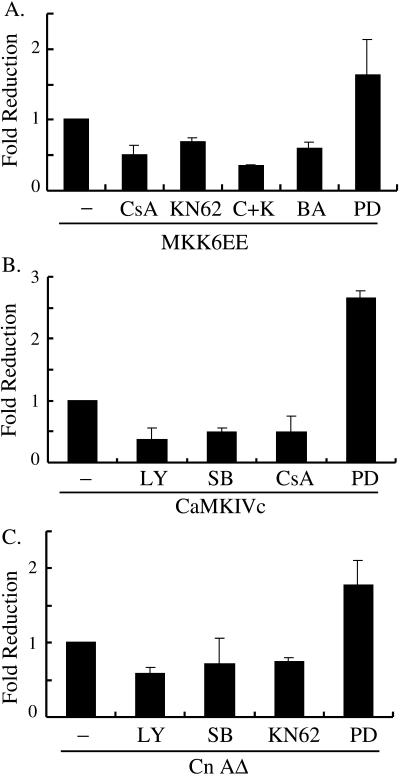
To further understand the relationship and cross talk among the three signaling pathways, we first cotransfected C2C12 cells with the myogenin reporter G133-luc and different activating signaling molecules. Drugs specifically blocking different pathways were added at the start of differentiation and the activity of G133-luc was measured 10 h after the addition of the drugs. The rational of this experiment was as follows: if molecule A acts upstream of molecule B, when a constitutively active A is transfected into cells, inhibition of endogenous B should reduce the stimulatory effect of A. In contrast, when a constitutively active B is transfected, inhibition of endogenous A should not affect the stimulatory effect of B. If A and B act on separate but nonredundant pathways, inhibition of either pathway would be expected to partially reduce the stimulatory effect mediated by the other pathway. As shown in Figure 6A, MKK6EE-mediated myogenin reporter activation could be partially blocked by drugs that either specifically inhibited CaMK (KN62), calcineurin (CsA), or chelated intracellular calcium (BAPTA/AM). Addition of KN62 and CsA together further reduced the promoter activation by MKK6EE. Similarly, activation of G133-luc by CaMKIVc could also be partially inhibited by CsA, SB202190, and LY294002, the latter two being inhibitors that specifically block p38 MAPK and PI3K, respectively, whereas the effect of CnAΔ could be partially inhibited by SB202190, LY294002, and KN62 (Figure 6, B and C). As a control, PD98059, which specifically inhibited the activation of the extracellular signal-regulated kinase pathway, failed to reduce the stimulatory effect of either MKK6EE, CaMKIVc, or CnAΔ. In contrast, PD98059 enhanced their stimulatory effect, in agreement with a negative role by extracellular signal-regulated kinase at the early phase of differentiation (Bennett and Tonks, 1997; Wu et al., 2000b). Collectively, these results suggest the p38 MAPK-, CaMK-, and calcineurin-mediated pathways act on separate pathways, yet all three are required to regulate myogenin expression.
Figure 6.
p38 MAPK-, CaMK-, and calcineurin-mediated pathways act on separate pathways. C2C12 cells were cotransfected with G133-luc in combination with either MKK6EE (A), CaMKIVc (B), or CnAΔ (C). Drugs were added at the time of media change from GM to DM and maintained for 10 h before determination of luciferase activity of each sample. Fold reduction was the ratio of luciferase activity in drug treated samples over that in the nontreated control. The experiments were done three times, and the mean ± SD is shown. The concentration of drugs was described in MATERIALS AND METHODS. LY, LY294002; SB, SB202190; BA, BAPTA/AM; PD, PD98059; C+K, both CsA and KN62 were added.
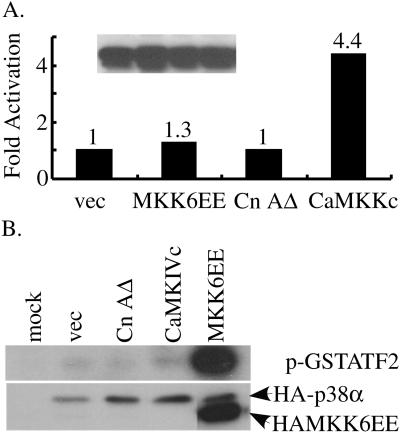
Because the cross talk between the CaMK- and p38 MAPK-mediated pathways was observed previously in neuronal cells (Enslen et al., 1996), we asked whether such a cross talk operated in C2C12 cells. We first cotransfected C2C12 cells with a His-tagged wild-type CaMKIV along with either an empty vector (negative control) or vectors encoding either MKK6EE, CnAΔ, or CaMKKc (the constitutively active upstream activator for CaMKIV, positive control) (Enslen et al., 1996). His-CaMKIV was pulled down (Figure 7A, see legend) and the kinase activity toward syntide-2 was then measured and quantified. Although CaMKKc activated CaMKIV as expected, both MKK6EE and CnAΔ failed to activate CaMKIV (Figure 7A). We then did a reverse experiment in which C2C12 cells were cotransfected with HA-p38α along with plasmids as indicated in Figure 7B. HA-p38α was immunoprecipitated and its kinase activity toward GST-ATF2 (1-92) was demonstrated. MKK6EE strongly activated p38α, whereas CaMKIVc and CnAΔ failed to do so. The above-mentioned experiments suggest CaMK and calcineurin do not significantly cross-activate p38 MAPK and that MKK6 and calcineurin do not significantly cross-activate CaMKIV in C2C12 cells. These data further strengthened our hypothesis that all three signaling pathways act on separate but nonredundant pathways to control myogenin expression.
Figure 7.
p38 MAPK- and calcineurin-mediated pathways do not activate CaMK, and the CaMK, calcineurin-mediated pathways do not activate p38 MAPK. (A) C2C12 cells were cotransfected with a vector encoding either His-CaMKIV (A) or HA-p38α (B) in combination with various plasmids as indicated. WCEs were prepared 24 h after transfection (in GM). (A) Level of His-CaMKIV was monitored by Western blot in the same order as shown in the bar graph (inset). His-CaMKIV from 50 μg of WCE was pulled down with Talon beads and subjected to in vitro kinase assays by using syntide-2 as the substrate. Radioactive counts were measured in a scintillation counter, and fold activation was the ratio of counts in samples transfected with the active signaling molecules over that with an empty vector. The experiment was done twice with similar results, and a representative is shown. (B) HA-p38α from 100 μg of WCE was immunoprecipitated and subjected to kinase assays by using GST-ATF2 (1-92) as the substrate. Proteins were resolved in SDS-PAGE and shown is an autoradiograph of the gel (top). The level of HA-p38α was monitored by Western blot (bottom).
CaMK and Calcineurin Fail to Activate MEF2 in RD Cells
We recently showed that both the p38 MAPK- and IGF/PI3K/Akt-mediated pathways were defective in Rhabdomyosarcoma-derived RD cells (Puri et al., 2000; Xu and Wu, 2000). To test whether CaMK and calcineurin were able to activate MyoD and MEF2s in RD cells, we cotransfected RD cells with different combinations of myogenic reporters and the activating signaling molecules. As shown in Figure 8A, although MKK6EE could significantly activate the MyoD-dependent (4RE-luc), MEF2-dependent (3MEF2-luc), and the native myogenin promoter (G133-luc, both MyoD- and MEF2-dependent) reporters as we showed previously (Xu and Wu, 2000), CaMK and calcineurin showed significantly decreased activation potential toward G133-luc (compare with Figure 4A). Interestingly, when we directly examined the effect of CaMK and calcineurin on MyoD, similar fold of activation of 4RE-luc was obtained compared with that in C2C12 cells, suggesting the effect of CaMK and calcineurin on MyoD was not compromised in RD cells (compare with Figure 5, B and C). In contrast, when we examined the effect of CaMK and calcineurin on MEF2, significant decrease in the activity of 3MEF2-luc was detected compared with that in C2C12 cells (compare with Figure 5A). We also directly compared the effect of CaMK and calcineurin on the transcriptional activity of MEF2C in RD vs. HeLa cells by using the vectors encoding gal4MEF2C fusion protein and a gal4-luc reporter. Although MKK6EE enhanced the transcriptional activity of gal4MEF2C in both RD and HeLa cells, CaMK and calcineurin failed to activate gal4MEF2C in RD cells as they did in HeLa cells (Figure 8B).
Figure 8.
CaMK and calcineurin barely activate MEF2 in RD cells. (A) RD cells were cotransfected with one of the three reporters (4RE-luc, 3MEF2-luc, and G133-luc) in combination with plasmids indicated in the inset box. Cells were let differentiate in DM for 48 h before harvest for luciferase activity determination. Fold activation was determined the same way as in the legend to Figure 4A. (B) RD or HeLa cells were cotransfected with gal4-luc and gal4MEF2C in combination with various plasmids as indicated. Luciferase activity was measured 24 h after cells were grown in DM, and fold induction determined as in A. (C) RD or HeLa cells were cotransfected with a vector encoding GFP-HDAC5 along with various plasmids as indicated. (D) RD cells were transfected with HDAC5-GFP and cells were grown in either GM or DM for 24 h. (C and D) Cells were fixed with methanol 24 h after transfection (in GM). More than 50 cells/sample were analyzed, and representative images were taken with a Nikon fluorescent microscope. (E) Model based on our data is presented. Signals from three separate but nonredundant pathways converge on three families of transcription factors: Six, MEF2, and MyoD, which in turn bind to the MEF3, MEF2, and the E box, respectively, in the myogenin proximal promoter and activate myogenin transcription. ∗, unlike the p38 MAPK and CaMKIV that can target the E box, and MEF2 and MEF3 sites in the myogenin promoter, calcineurin mainly targets the E box and the MEF2 site without an obvious effect on the MEF3 site. Nevertheless, calcineurin is still capable of activating a Six-dependent MEF3 reporter (3xMEF3-luc), suggesting calcineurin is able to target the MEF3 site in promoters other than that of myogenin.
Recently, the CaMK-mediated pathway was shown to target HDAC5, resulting in its dissociation from MEF2s, shuttling from the nucleus to cytoplasm and the subsequent transcriptional activation of MEF2s (Lu et al., 2000a,b; McKinsey et al., 2000a,b). We asked whether the reduced activation of MEF2s by CaMK in RD cells was caused by defective cytoplasmic shuttling of HDAC5. To this end, we cotransfected RD or HeLa cells with a vector encoding HDAC5-GFP along with either an empty vector or various active signaling molecules. In agreement with the previous report, only the constitutively active CaMKIV efficiently shuttled HDAC5-GFP from the nucleus into cytoplasm in HeLa cells (Figure 8C, top) (McKinsey et al., 2000a). The constitutively active CnAΔ and MKK6EE did not have such an effect. To our surprise, CaMK could also shuttle HDAC5-GFP into the cytoplasm in RD cells as efficiently as in HeLa cells (Figure 8C, bottom). This strongly argues that cytoplasmic shuttling of HDAC5 is not the only mechanism by which CaMK activates MEF2s.
Because the constitutively active CaMKIV was used in the above-mentioned experiment, it was still unclear whether the endogenous CaMK was properly activated in response to the differentiation signals in RD cells. To address this issue, we used HDAC5-GFP cytoplasmic shuttling as a functional readout for activation of the endogenous CaMK. As seen in normal C2C12 myogenic cells (Figure 3B), HDAC5-GFP was present solely in the nucleus in GM and was predominantly cytoplasmic in DM (Figure 8D), suggesting the signaling pathway leading to CaMK activation in response to differentiation signals was not defective in RD cells.
DISCUSSION
Multiple Intracellular Signal Transduction Pathways Modulate Myogenesis by Increasing Myogenin Gene Transcription
It is well recognized that myogenin plays a key role in executing muscle differentiation program, because loss of myogenin causes severe muscle defect without affecting the formation and positioning of myoblasts (Hasty et al., 1993; Nabeshima et al., 1993). Moreover, overexpression of MyoD in embryoid bodies derived from myogenin null ES cells fails to rescue the differentiation defect, suggesting a unique role for myogenin in differentiation (Myer et al., 2001). We recently showed the IGF/PI3K/Akt pathway enhances myogenesis by transcriptionally up-regulating myogenin mRNA level (Xu and Wu, 2000). Herein, we demonstrate that the p38 MAPK and two calcium/calmodulin-activated pathways (i.e., CaMK and calcineurin) also positively regulate myogenesis by increasing myogenin gene transcription. A model summarizing our current data is presented (Figure 8E). In addition, many stimuli known to affect myogenesis were also shown to affect the myogenin expression level (Huang et al., 2000; Rochard et al., 2000). Thus, the myogenin gene seems to serve as one of the main regulatory targets in myogenic cells in response to various pro- or counterdifferentiation cues.
Calcium Signal Is Required for Myogenin Induction
Because addition of BAPTA/AM inhibits myogenin expression (Figure 1D), it indicates that the calcium signal is required for myogenin expression. To find out whether it is the influx of extracellular calcium through the plasma membrane calcium channels or the calcium released from internal calcium stores (e.g., endoplasmic reticulum) that is responsible for the initial calcium signal, we checked the effect of nifedipine, a specific blocker for the L-type membrane calcium channels. The fact that nifedipine can significantly inhibit myogenin induction suggests the influx of extracellular calcium plays a key role in myogenin induction (Figure 1B), in agreement with a recent report (Friday et al., 2000). However, this result does not rule out a role for the calcium released from internal calcium stores. It is possible that the calcium influx from extracellular environment sends out the initial signal, and this can then induce the calcium release from the internal calcium stores (via inositol 1,4,5-trisphosphate receptor and the ryanodine receptor), which may in turn augment and prolong the initial calcium signal (Mikoshiba, 1997; Berchtold et al., 2000).
CaMK- and calcineurin-mediated pathways are identified as two main cellular signaling pathways transmitting the calcium signals (Klee et al., 1998; Soderling, 1999; Olson and Williams, 2000a,b). Although CaMKIV is used in this study and other reports, the exact isoform(s) that function in muscle cells to regulate myogenin expression remains to be identified. One piece of supporting evidence for a role of CaMKIV in activating transcription is that CaMKIV mainly localizes in the nucleus, whereas CaMKI is mainly cytosolic (Soderling, 1999).
Stimulatory Signals from Multiple Pathways Converge on MyoD, MEF2s, and Six Proteins
Like the IGF/PI3K/Akt pathway, the p38 MAPK-, CaMK-, and calcineurin-mediated pathways also regulate myogenin expression by enhancing the transcriptional activity of MyoD and MEF2s, two families of transcription factors indispensable for muscle differentiation. In C2C12 cells, Myf5 mRNA and protein are not detected, making MyoD the only possible candidate in the MRF family that participates in myogenin regulation. Because Myf5 is the first MRF expressed during myogenesis and it is also the only MRF present in proliferating myoblasts of certain myogenic cell lines (e.g., rat L6 cells), it is reasonable to postulate that Myf5 could also be a potential nuclear signal receiver in response to various signaling pathways controlling myogenin expression. Whether this is the case remains to be experimentally tested.
In addition to MyoD and MEF2s, for the first time, we are able to demonstrate that the homeodomain-containing Six proteins can also be activated by the p38 MAPK-, CaMK-, and calcineurin-mediated pathways (Figure 8E). Although the MEF3 site in the myogenin promoter does not respond to calcineurin very well (Figure 4B), calcineurin can activate a Six-dependent multimerized MEF3 reporter (Figure 5E). It is possible that, even though activation of Six by calcineurin is not critical in myogenin expression, it may be required for the regulation of other genes containing the MEF3 site. Among members of the Six family, Six1 and 4 are known to be expressed in somites and myogenic cell lines. Whether these are the actual Six proteins involved in myogenin regulation remains to be established. Because Six family proteins are well conserved from Drosophila to mammals and are implicated in many developmental processes (ranging from eye formation, muscle differentiation to forebrain formation), further analysis is needed to understand the detailed mechanisms by which the three signaling pathways regulate Sixs and their associated proteins (e.g., Eya and Dach). These studies will be instrumental to the elucidation of the above-mentioned biological processes.
Although the three pathways are able to activate Six proteins bound to the MEF3 site, MyoD and MEF2 seem to be the main targets, because simultaneous deletion of the E box and the MEF2 site severely reduces the responsiveness of the myogenin promoter to all three signaling pathways (Figure 4B). It is also interesting to note that, unlike MKK6 (which not only activates MEF2 but also significantly activates MyoD) (Wu et al., 2000b), CaMK and calcineurin seem to preferentially target MEF2s (Figures 4B and 5, A and B). However, data from Figure 4B (in which G133MEF2 could still be activated by CaMK and calcineurin) and Figure 5 strongly argue that MyoD is another downstream target. In support of our notion, it has recently been shown that the constitutively active calcineurin significantly enhances myogenic conversion of 10T1/2 fibroblasts induced by a stably transfected MyoD (Delling et al., 2000).
At present, we favor the model in which CaMK and calcineurin do not directly bind MyoD and MEF2, because we fail to detect direct interaction in either coimmunoprecipitation experiments or yeast two-hybrid assays (our unpublished data). In vitro, CaMK is shown to directly phosphorylate MEF2D and HDAC5 (Blaeser et al., 2000; McKinsey et al., 2000a). However, convincing in vivo phosphorylation and binding data are still lacking at present. As to the effect of calcineurin on MEF2s, it is still controversial whether this effect is dependent on nuclear factor of activated T cells (NFATs), the well-documented calcineurin substrates in lymphocytes. Two studies carried out in lymphocytes suggest NFATs can augment and mediate the effect of calcineurin on MEF2s, even in the absence of direct binding to the cognate NFAT sites (Blaeser et al., 2000; Youn et al., 2000). In addition, physical interaction between NFATs and MEF2s has also been demonstrated (Blaeser et al., 2000; Youn et al., 2000). However, it is not known whether such a mechanism exists in myogenic cells, and it has not been clearly demonstrated whether activation of MEF2s by calcineurin absolutely requires NFATs. An MEF2 mutant unable to interact with NFATs will be useful to address this question. In one report carried out in muscle cells, it shows an independent but cooperative relationship between NFATs and MEF2s in response to calcineurin in determining muscle fiber types (Wu et al., 2000a). As to a general role for NFATs in early myogenic differentiation, conflicting data exist. One report indicates NFATc3 can mediate the stimulatory effect of calcineurin on myogenic differentiation (Delling et al., 2000), whereas the other report completely rules out a role for NFAT in early muscle differentiation and myogenin induction in response to calcineurin (Friday et al., 2000). Thus, the mechanism by which calcineurin activates MEF2s and the role of NFATs in myogenic differentiation and MEF2 activation by calcineurin remain to be clarified.
Functional Status of CaMK- and Calcineurin-mediated Pathways in RD Cells
We showed previously that both the p38 MAPK and the IGF/PI3K/Akt pathways were defective in Rhabdomyosarcoma-derived RD cells (Puri et al., 2000; Xu and Wu, 2000). Interestingly, when tested in RD cells, the ability of CaMK or calcineurin to activate MyoD is as good as that in normal C2C12 cells, whereas their effects on MEF2 are greatly compromised compared with that in C2C12 cells (Figures 5, A and B, and 8, A and B). The mechanism by which CaMK activates MEF2s has been shown to involve HDAC5 translocation from the nucleus to cytoplasm (Lu et al., 2000a,b; McKinsey et al., 2000a,b). In the cytoplasm, 14-3-3 proteins bind the phosphorylated HDAC5 and retain it in the cytosol (Grozinger and Schreiber, 2000; McKinsey et al., 2000b; Wang et al., 2000). In addition to HDAC5, MEF2s are also known to be bound and repressed by many other transcriptional repressors (e.g., Cabin 1, MITR, and HDAC4 and 7) (Sparrow et al., 1999; Youn and Liu, 2000; Dressel et al., 2001). It is likely that the association of MEF2s with these different repressors is cell type and target gene dependent. Regarding the role of HDAC5, one unanswered question is whether HDAC5 cytoplasmic translocation is necessary and sufficient for subsequent activation of the MEF2 transcriptional activity. The defective MEF2 activation by CaMK in RD cells provides a nice system for us to address a part of this question. To our surprise, even though GFP-HDAC5 can be shuttled to cytoplasm by the active CaMK in RD cells as efficiently as in other cells, MEF2s remain inactive. This indicates that at least two signals are required for CaMK to activate MEF2s: one directly dependent on CaMK involving HDAC5 cytoplasmic translocation. The other signal can be either CaMK dependent (e.g., CaMK may help recruit a transcriptional coactivator to MEF2s) or CaMK independent (e.g., a signal from another pathway is also required). Because HDAC5 efficiently shuttles from the nucleus to cytoplasm upon differentiation (Figure 8D), it suggests that the endogenous CaMK can be properly activated in RD cells in response to differentiation cues and that the CaMK pathway per se is normal in RD cells. Our results further suggest that HDAC5 cytoplasmic shuttling is necessary but not sufficient for CaMK to activate MEF2s.
ACKNOWLEDGMENTS
We thank Drs. T.R. Soderling, P.L. Puri, J. Han, D.C. Chang, and A.L. Miller for reagents. We also thank Carol Wong for technical help. This project was supported by grants HKUST6205/00 M and HKUST6102/01 M from Hong Kong Research Grant Council and by the Areas of Excellence scheme established under the University Grants Committee of the Hong Kong Special Administrative Region, China (Project No. AoE/B-15/01).
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.02–02–0016. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.02–02–0016.
REFERENCES
- Arnold HH, Winter B. Muscle differentiation: more complexity to the network of myogenic regulators. Curr Opin Genet Dev. 1998;8:539–544. doi: 10.1016/s0959-437x(98)80008-7. [DOI] [PubMed] [Google Scholar]
- Bennett AM, Tonks NK. Regulation of distinct stages of skeletal muscle differentiation by mitogen-activated protein kinases. Science. 1997;278:1288–1291. doi: 10.1126/science.278.5341.1288. [DOI] [PubMed] [Google Scholar]
- Berchtold MW, Brinkmeier H, Muntener M. Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol Rev. 2000;80:1215–1265. doi: 10.1152/physrev.2000.80.3.1215. [DOI] [PubMed] [Google Scholar]
- Blaeser F, Ho N, Prywes R, Chatila TA. Ca(2+)-dependent gene expression mediated by MEF2 transcription factors. J Biol Chem. 2000;275:197–209. doi: 10.1074/jbc.275.1.197. [DOI] [PubMed] [Google Scholar]
- Coolican SA, Samuel DS, Ewton DZ, McWade FJ, Florini JR. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem. 1997;272:6653–6662. doi: 10.1074/jbc.272.10.6653. [DOI] [PubMed] [Google Scholar]
- Cuenda A, Cohen P. Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J Biol Chem. 1999;274:4341–4346. doi: 10.1074/jbc.274.7.4341. [DOI] [PubMed] [Google Scholar]
- Delling U, Tureckova J, Lim HW, De Windt LJ, Rotwein P, Molkentin JD. A calcineurin-NFATc3-dependent pathway regulates skeletal muscle differentiation and slow myosin heavy-chain expression. Mol Cell Biol. 2000;20:6600–6611. doi: 10.1128/mcb.20.17.6600-6611.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressel U, Bailey PJ, Wang SC, Downes M, Evans RM, Muscat GE. A dynamic role for HDAC7 in MEF2-mediated muscle differentiation. J Biol Chem. 2001;276:17007–17013. doi: 10.1074/jbc.M101508200. [DOI] [PubMed] [Google Scholar]
- Enslen H, Tokumitsu H, Stork PJ, Davis RJ, Soderling TR. Regulation of mitogen-activated protein kinases by a calcium/calmodulin-dependent protein kinase cascade. Proc Natl Acad Sci USA. 1996;93:10803–10808. doi: 10.1073/pnas.93.20.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florini JR, Ewton DZ, Roof SL. Insulin-like growth factor-I stimulates terminal myogenic differentiation by induction of myogenin gene expression. Mol Endocrinol. 1991;5:718–724. doi: 10.1210/mend-5-5-718. [DOI] [PubMed] [Google Scholar]
- Friday BB, Horsley V, Pavlath GK. Calcineurin activity is required for the initiation of skeletal muscle differentiation. J Cell Biol. 2000;149:657–666. doi: 10.1083/jcb.149.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozinger CM, Schreiber SL. Regulation of histone deacetylase 4, and 5, and transcriptional activity by 14-3-3-dependent cellular localization. Proc Natl Acad Sci USA. 2000;97:7835–7840. doi: 10.1073/pnas.140199597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Jiang Y, Li Z, Kravchenko VV, Ulevitch RJ. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature. 1997;386:296–299. doi: 10.1038/386296a0. [DOI] [PubMed] [Google Scholar]
- Hasty P, Bradley A, Morris JH, Edmondson DG, Venuti JM, Olson EN, Klein WH. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene [see comments] Nature. 1993;364:501–506. doi: 10.1038/364501a0. [DOI] [PubMed] [Google Scholar]
- Heanue TA, Reshef R, Davis RJ, Mardon G, Oliver G, Tomarev S, Lassar AB, Tabin CJ. Synergistic regulation of vertebrate muscle development by Dach2, Eya2, and Six1, homologs of genes required for Drosophila eye formation. Genes Dev. 1999;13:3231–3243. doi: 10.1101/gad.13.24.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Li J, Zhang Y, Wu C. The roles of integrin-linked kinase in the regulation of myogenic differentiation. J Cell Biol. 2000;150:861–872. doi: 10.1083/jcb.150.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K, Sato S, Ozaki H, Ikeda K. Six family genes–structure, and function as transcription factors, and their roles in development. Bioessays. 2000;22:616–626. doi: 10.1002/1521-1878(200007)22:7<616::AID-BIES4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Klee CB, Ren H, Wang X. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem. 1998;273:13367–13370. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- Lassar AB, Skapek SX, Novitch B. Regulatory mechanisms that coordinate skeletal muscle differentiation and cell cycle withdrawal. Curr Opin Cell Biol. 1994;6:788–794. doi: 10.1016/0955-0674(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Lu J, McKinsey TA, Nicol RL, Olson EN. Signal-dependent activation of the MEF2 transcription factor by dissociation from histone deacetylases. Proc Natl Acad Sci USA. 2000a;97:4070–4075. doi: 10.1073/pnas.080064097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, McKinsey TA, Zhang CL, Olson EN. Regulation of skeletal myogenesis by association of the MEF2 transcription factor with class II histone deacetylases. Mol Cell. 2000b;6:233–244. doi: 10.1016/s1097-2765(00)00025-3. [DOI] [PubMed] [Google Scholar]
- McKinsey TA, Zhang CL, Lu J, Olson EN. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature. 2000a;408:106–111. doi: 10.1038/35040593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey TA, Zhang CL, Olson EN. Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14-3-3 to histone deacetylase 5. Proc Natl Acad Sci USA. 2000b;97:14400–14405. doi: 10.1073/pnas.260501497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikoshiba K. The InsP3 receptor and intracellular Ca2+ signaling. Curr Opin Neurobiol. 1997;7:339–345. doi: 10.1016/s0959-4388(97)80061-x. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Black BL, Martin JF, Olson EN. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell. 1995;83:1125–1136. doi: 10.1016/0092-8674(95)90139-6. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Olson EN. Defining the regulatory networks for muscle development. Curr Opin Genet Dev. 1996;6:445–453. doi: 10.1016/s0959-437x(96)80066-9. [DOI] [PubMed] [Google Scholar]
- Myer A, Olson EN, Klein WH. MyoD cannot compensate for the absence of myogenin during skeletal muscle differentiation in murine embryonic stem cells. Dev Biol. 2001;229:340–350. doi: 10.1006/dbio.2000.9985. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y, Hanaoka K, Hayasaka M, Esumi E, Li S, Nonaka I. Myogenin gene disruption results in perinatal lethality because of severe muscle defect [see comments] Nature. 1993;364:532–535. doi: 10.1038/364532a0. [DOI] [PubMed] [Google Scholar]
- Ohto H, Kamada S, Tago K, Tominaga SI, Ozaki H, Sato S, Kawakami K. Cooperation of six and eya in activation of their target genes through nuclear translocation of Eya. Mol Cell Biol. 1999;19:6815–6824. doi: 10.1128/mcb.19.10.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EN, Williams RS. Calcineurin signaling, and muscle remodeling. Cell. 2000a;101:689–692. doi: 10.1016/s0092-8674(00)80880-6. [DOI] [PubMed] [Google Scholar]
- Olson EN, Williams RS. Remodeling muscles with calcineurin. Bioessays. 2000b;22:510–519. doi: 10.1002/1521-1878(200011)22:11<1049::AID-BIES14>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Pignoni F, Hu B, Zavitz KH, Xiao J, Garrity PA, Zipursky SL. The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development. Cell. 1997;91:881–891. doi: 10.1016/s0092-8674(00)80480-8. [DOI] [PubMed] [Google Scholar]
- Puri PL, Sartorelli V. Regulation of muscle regulatory factors by DNA-binding, interacting proteins, and post-transcriptional modifications. J Cell Physiol. 2000;185:155–173. doi: 10.1002/1097-4652(200011)185:2<155::AID-JCP1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Puri PL, Wu Z, Zhang P, Wood LD, Bhakta KS, Han J, Feramisco JR, Karin M, Wang JY. Induction of terminal differentiation by constitutive activation of p38 MAP kinase in human rhabdomyosarcoma cells. Genes Dev. 2000;14:574–584. [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Buckingham M. From insect eye to vertebrate muscle: redeployment of a regulatory network. Genes Dev. 1999;13:3171–3178. doi: 10.1101/gad.13.24.3171. [DOI] [PubMed] [Google Scholar]
- Rochard P, Rodier A, Casas F, Cassar-Malek I, Marchal-Victorion S, Daury L, Wrutniak C, Cabello G. Mitochondrial activity is involved in the regulation of myoblast differentiation through myogenin expression, and activity of myogenic factors. J Biol Chem. 2000;275:2733–2744. doi: 10.1074/jbc.275.4.2733. [DOI] [PubMed] [Google Scholar]
- Soderling TR. The Ca-calmodulin-dependent protein kinase cascade. Trends Biochem Sci. 1999;24:232–236. doi: 10.1016/s0968-0004(99)01383-3. [DOI] [PubMed] [Google Scholar]
- Sparrow DB, Miska EA, Langley E, Reynaud-Deonauth S, Kotecha S, Towers N, Spohr G, Kouzarides T, Mohun TJ. MEF-2 function is modified by a novel co-repressor, MITR. EMBO J. 1999;18:5085–5098. doi: 10.1093/emboj/18.18.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz F, Demignon J, Porteu A, Kahn A, Concordet JP, Daegelen D, Maire P. Expression of myogenin during embryogenesis is controlled by Six/sine oculis homeoproteins through a conserved MEF3 binding site. Proc Natl Acad Sci USA. 1998;95:14220–14225. doi: 10.1073/pnas.95.24.14220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir Y, Bengal E. Phosphoinositide 3-kinase induces the transcriptional activity of MEF2 proteins during muscle differentiation. J Biol Chem. 2000;275:34424–34432. doi: 10.1074/jbc.M005815200. [DOI] [PubMed] [Google Scholar]
- Wang AH, Kruhlak MJ, Wu J, Bertos NR, Vezmar M, Posner BI, Bazett-Jones DP, Yang XJ. Regulation of histone deacetylase 4 by binding of 14-3-3 proteins. Mol Cell Biol. 2000;20:6904–6912. doi: 10.1128/mcb.20.18.6904-6912.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werlen G, Jacinto E, Xia Y, Karin M. Calcineurin preferentially synergizes with PKC-theta to activate JNK and IL-2 promoter in T lymphocytes. EMBO J. 1998;17:3101–3111. doi: 10.1093/emboj/17.11.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, et al. MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type. EMBO J. 2000a;19:1963–1973. doi: 10.1093/emboj/19.9.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Woodring PJ, Bhakta KS, Tamura K, Wen F, Feramisco JR, Karin M, Wang JY, Puri PL. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol Cell Biol. 2000b;20:3951–3964. doi: 10.1128/mcb.20.11.3951-3964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wu Z. The insulin-like growth factor-phosphatidylinositol 3-kinase-Akt signaling pathway regulates myogenin expression in normal myogenic cells but not in rhabdomyosarcoma-derived RD cells. J Biol Chem. 2000;275:36750–36757. doi: 10.1074/jbc.M005030200. [DOI] [PubMed] [Google Scholar]
- Yee SP, Rigby PW. The regulation of myogenin gene expression during the embryonic development of the mouse. Genes Dev. 1993;7:1277–1289. doi: 10.1101/gad.7.7a.1277. [DOI] [PubMed] [Google Scholar]
- Youn HD, Chatila TA, Liu JO. Integration of calcineurin, and MEF2 signals by the coactivator p300 during T-cell apoptosis. EMBO J. 2000;19:4323–4331. doi: 10.1093/emboj/19.16.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn HD, Liu JO. Cabin1 represses MEF2-dependent Nur77 expression, and T cell apoptosis by controlling association of histone deacetylases, and acetylases with MEF2. Immunity. 2000;13:85–94. doi: 10.1016/s1074-7613(00)00010-8. [DOI] [PubMed] [Google Scholar]
- Yun K, Wold B. Skeletal muscle determination and differentiation: story of a core regulatory network and its context. Curr Opin Cell Biol. 1996;8:877–889. doi: 10.1016/s0955-0674(96)80091-3. [DOI] [PubMed] [Google Scholar]
- Zetser A, Gredinger E, Bengal E. p38 mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. J Biol Chem. 1999;274:5193–5200. doi: 10.1074/jbc.274.8.5193. [DOI] [PubMed] [Google Scholar]
- Zhao M, New L, Kravchenko VV, Kato Y, Gram H, di Padova F, Olson EN, Ulevitch RJ, Han J. Regulation of the MEF2 family of transcription factors by p38. Mol Cell Biol. 1999;19:21–30. doi: 10.1128/mcb.19.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]