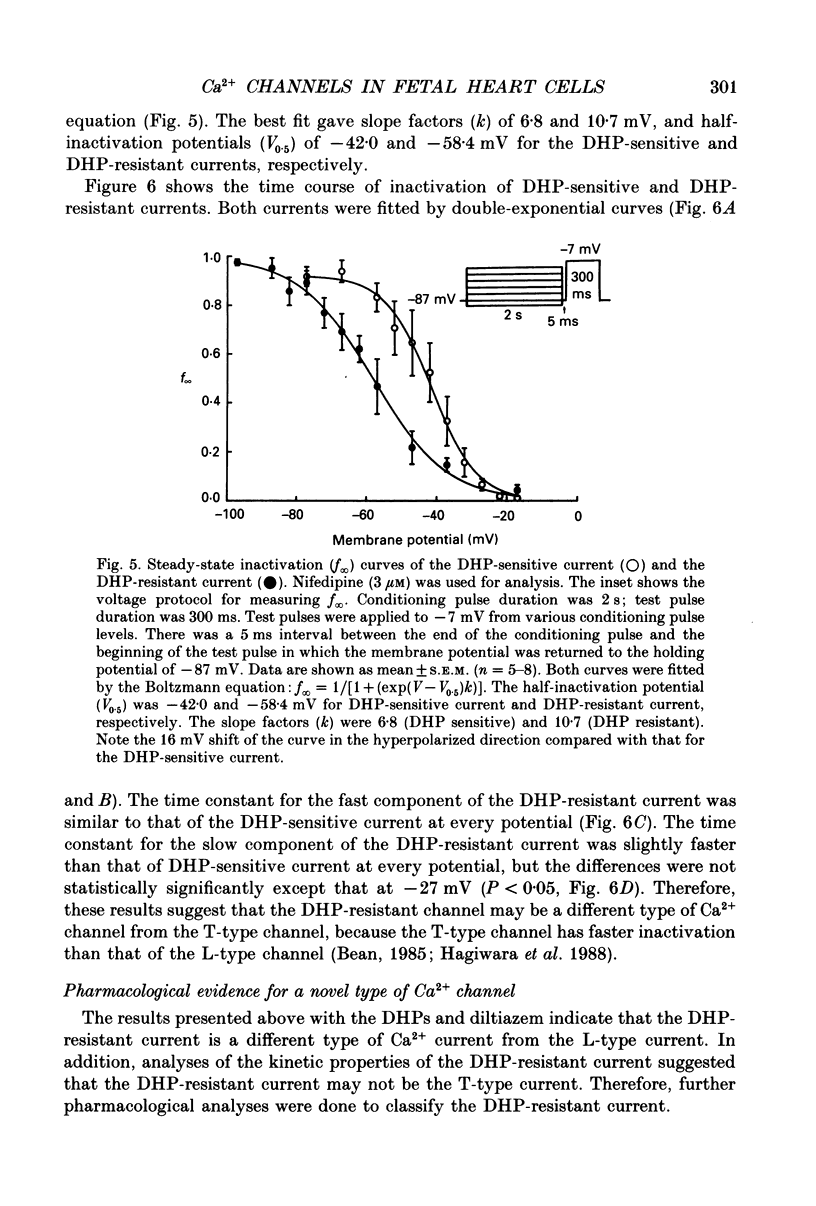
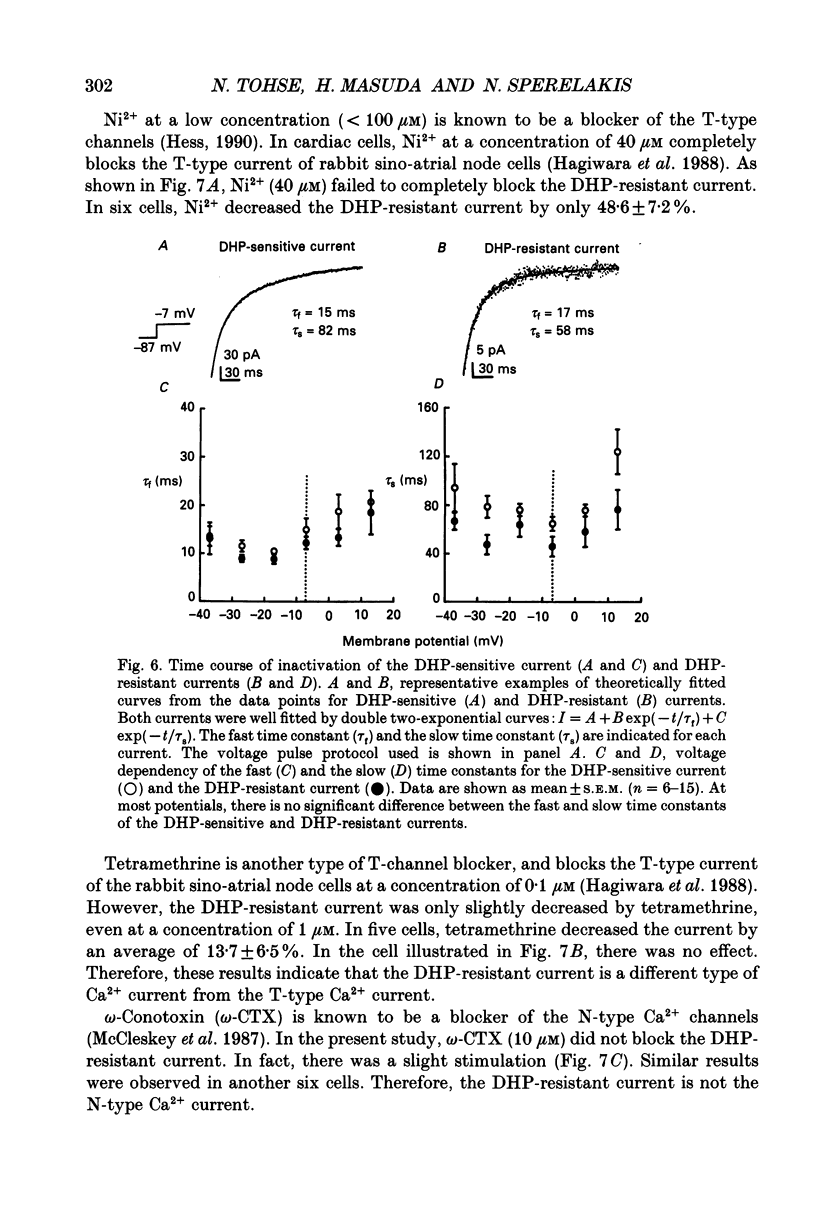
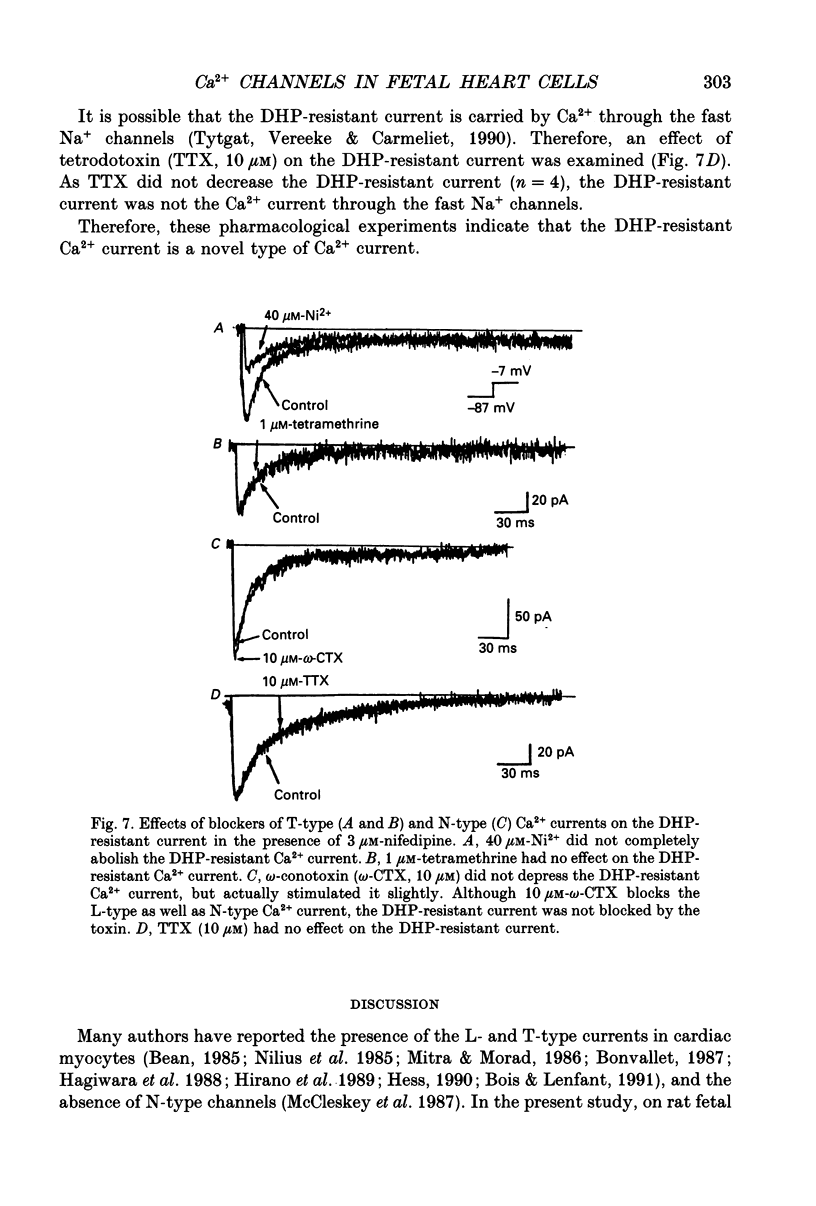
Abstract
1. Single cardiomyocytes of 18-day-old rat fetuses were isolated to characterize the cardiac Ca2+ channels in the fetal period, using whole-cell voltage clamp (Na+, K(+)-free external solution and K(+)-free internal solution), and depolarizing test pulses from a holding potential (HP) of -87 mV were applied. 2. The Ca2+ current was completely blocked by 2 mM-CO2+, but not completely blocked by the dihydropyridine (DHP) Ca2+ antagonist nifedipine. Nifedipine (3 microM) decreased the amplitude of the current (at -7 mV) by 65.9 +/- 3.4% (n = 20). At a HP of -47 mV, nifedipine decreased the Ca2+ current to about the same degree. Diltiazem (1 microM) did not block the nifedipine-resistant current which remained. 3. Nitrendipine, another DHP Ca2+ antagonist, had effects on the Ca2+ current similar to those of nifedipine. 4. The DHP-resistant current was not blocked by T-type channel blockers (Ni2+, tetramethrine) or an N-type blocker (omega-conotoxin). 5. In conclusion, rat fetal cardiomyocytes may have a unique type of Ca2+ channel (ICa(fe)), which decreases in amplitude and becomes less prominent during subsequent development.
Full text
PDF

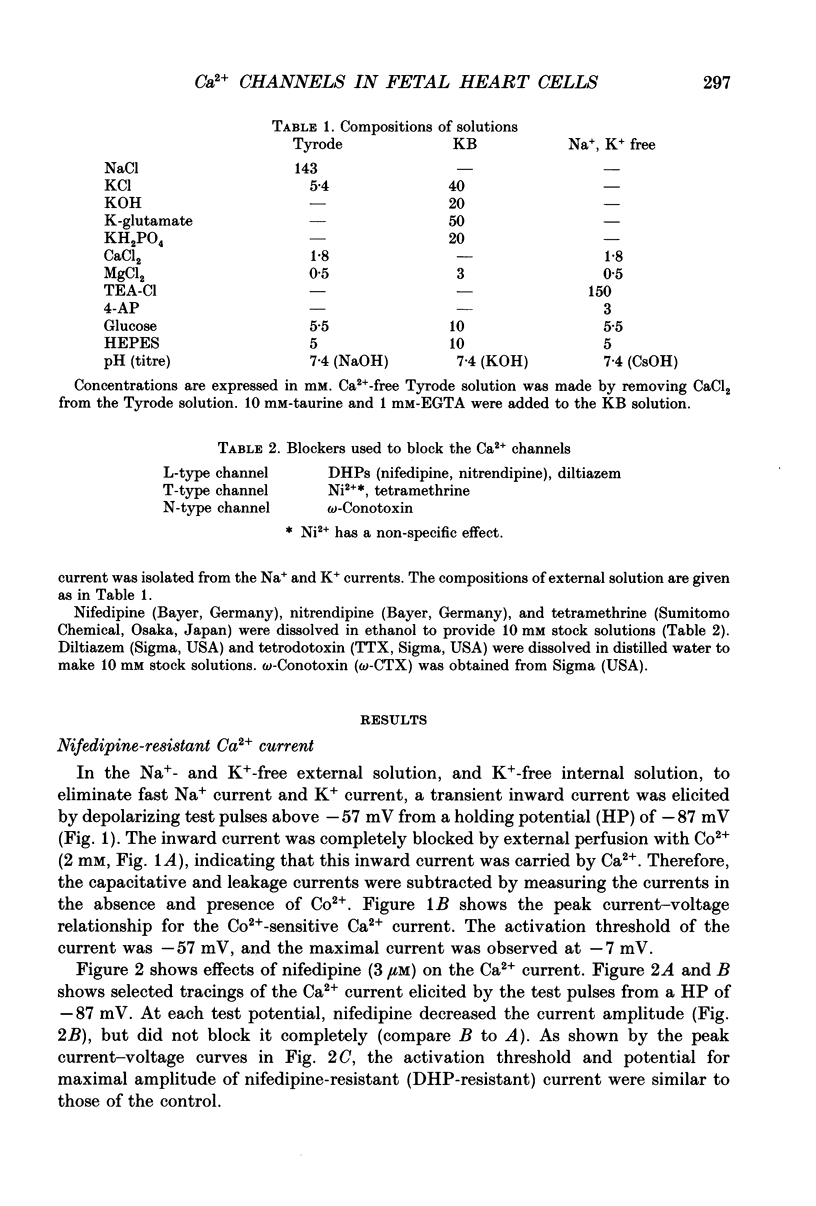
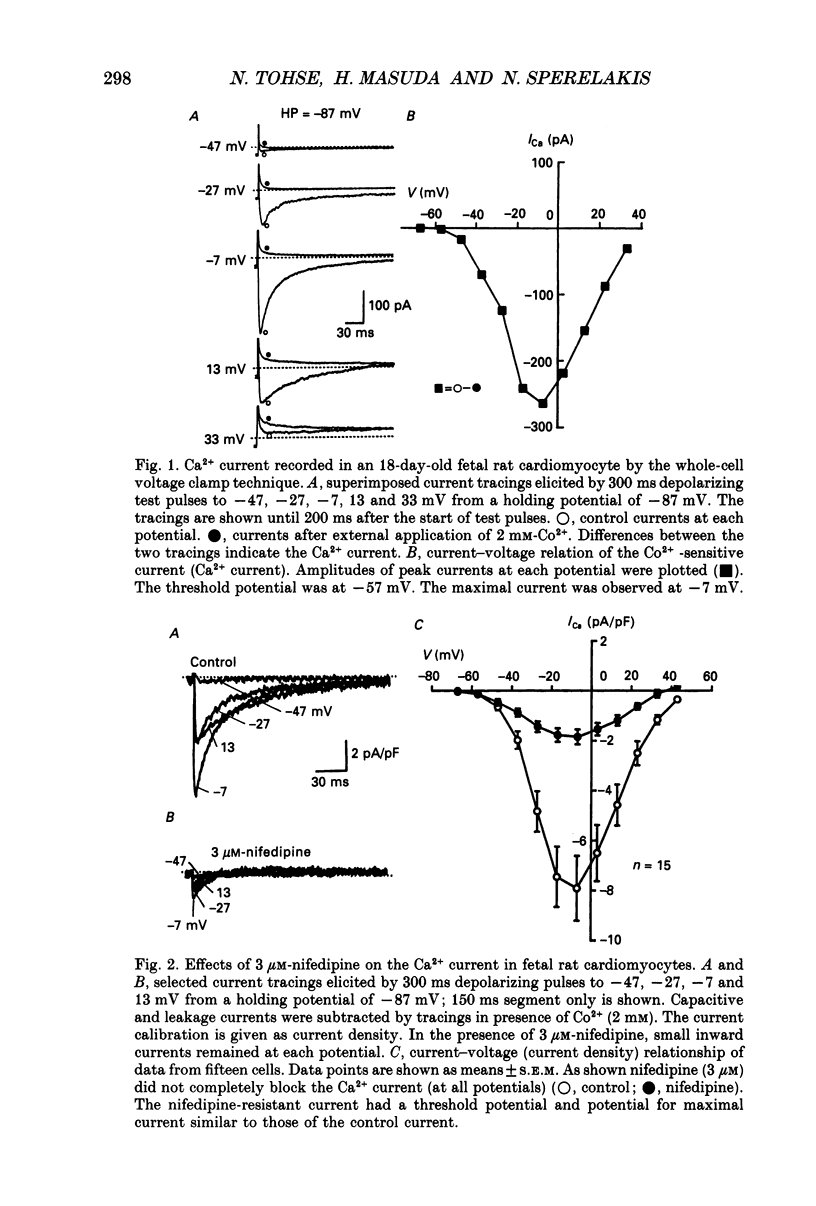
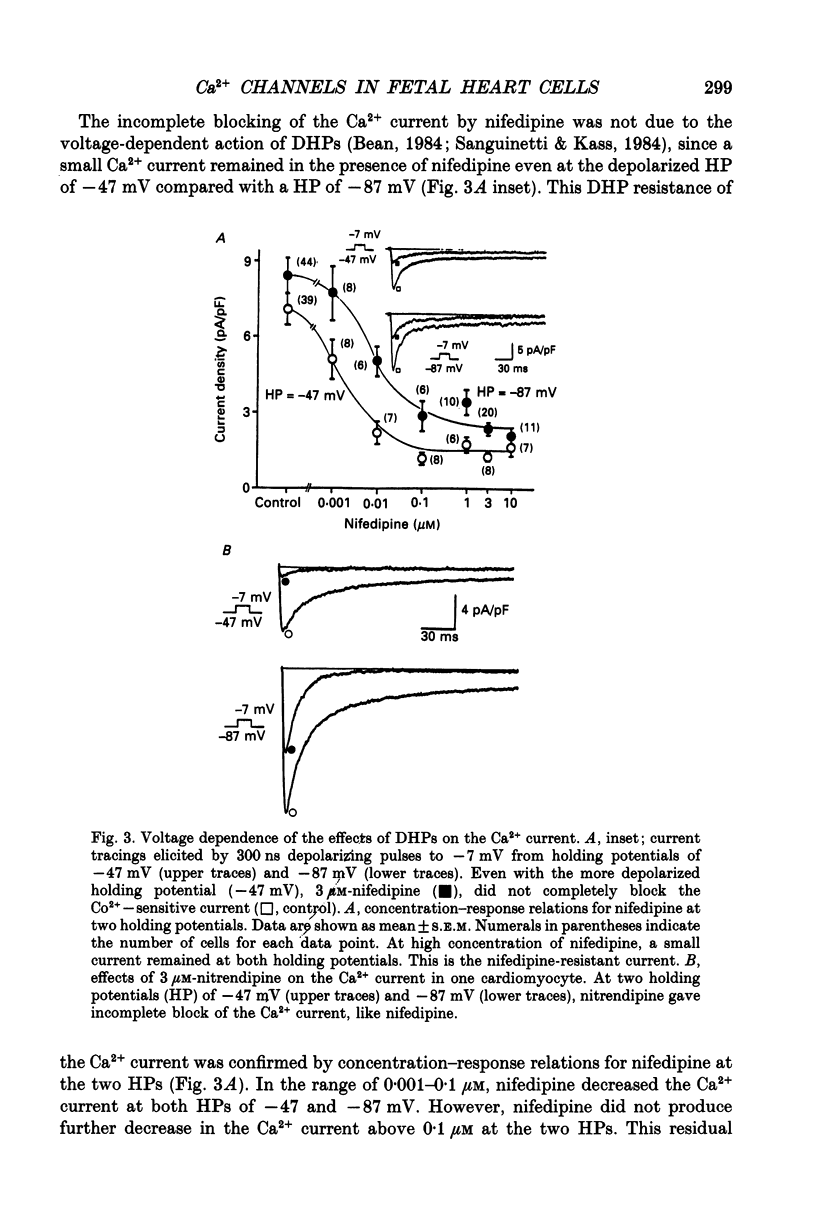
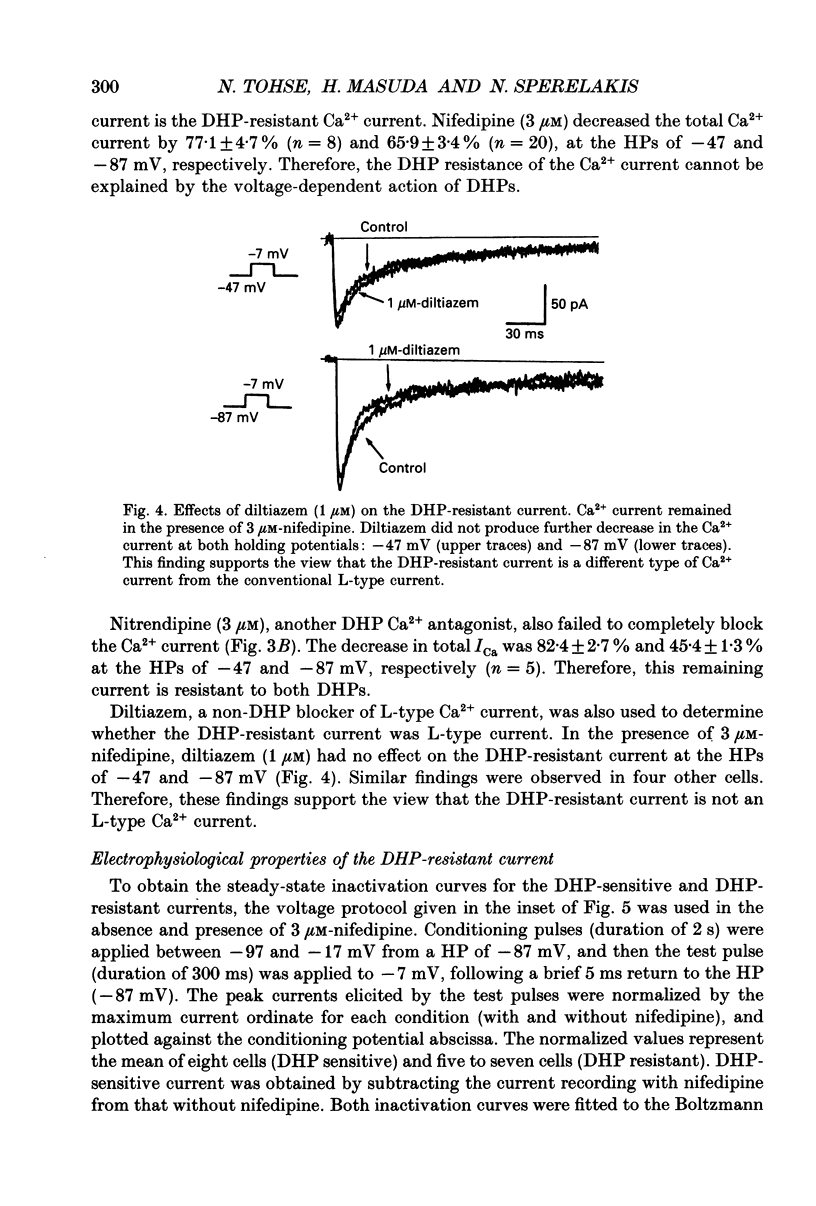



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bois P., Lenfant J. Evidence for two types of calcium currents in frog cardiac sinus venosus cells. Pflugers Arch. 1991 Feb;417(6):591–596. doi: 10.1007/BF00372956. [DOI] [PubMed] [Google Scholar]
- Bonvallet R. A low threshold calcium current recorded at physiological Ca concentrations in single frog atrial cells. Pflugers Arch. 1987 May;408(5):540–542. doi: 10.1007/BF00585084. [DOI] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H., Kameyama M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J Physiol. 1988 Jan;395:233–253. doi: 10.1113/jphysiol.1988.sp016916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y., Fozzard H. A., January C. T. Characteristics of L- and T-type Ca2+ currents in canine cardiac Purkinje cells. Am J Physiol. 1989 May;256(5 Pt 2):H1478–H1492. doi: 10.1152/ajpheart.1989.256.5.H1478. [DOI] [PubMed] [Google Scholar]
- Hume J. R. Comparative interactions of organic Ca++ channel antagonists with myocardial Ca++ and K+ channels. J Pharmacol Exp Ther. 1985 Jul;234(1):134–140. [PubMed] [Google Scholar]
- Kallen R. G., Sheng Z. H., Yang J., Chen L. Q., Rogart R. B., Barchi R. L. Primary structure and expression of a sodium channel characteristic of denervated and immature rat skeletal muscle. Neuron. 1990 Feb;4(2):233–242. doi: 10.1016/0896-6273(90)90098-z. [DOI] [PubMed] [Google Scholar]
- Kass R. S. Nisoldipine: a new, more selective calcium current blocker in cardiac Purkinje fibers. J Pharmacol Exp Ther. 1982 Nov;223(2):446–456. [PubMed] [Google Scholar]
- Kawano S., DeHaan R. L. Low-threshold current is major calcium current in chick ventricle cells. Am J Physiol. 1989 May;256(5 Pt 2):H1505–H1508. doi: 10.1152/ajpheart.1989.256.5.H1505. [DOI] [PubMed] [Google Scholar]
- Kawashima Y., Ochi R. Voltage-dependent decrease in the availability of single calcium channels by nitrendipine in guinea-pig ventricular cells. J Physiol. 1988 Aug;402:219–235. doi: 10.1113/jphysiol.1988.sp017201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M., Ishima T., Taniguchi N., Kimura K., Sada H., Sperelakis N. Developmental changes in beta-adrenoceptors, muscarinic cholinoceptors and Ca2+ channels in rat ventricular muscles. Br J Pharmacol. 1990 Feb;99(2):334–339. doi: 10.1111/j.1476-5381.1990.tb14704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Lin J. W., Cherksey B. Blocking and isolation of a calcium channel from neurons in mammals and cephalopods utilizing a toxin fraction (FTX) from funnel-web spider poison. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1689–1693. doi: 10.1073/pnas.86.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangos P. J., Sperelakis N., Patel J. Ontogeny of calcium antagonist binding sites in chick brain and heart. J Neurochem. 1984 May;42(5):1338–1342. doi: 10.1111/j.1471-4159.1984.tb02792.x. [DOI] [PubMed] [Google Scholar]
- McCleskey E. W., Fox A. P., Feldman D. H., Cruz L. J., Olivera B. M., Tsien R. W., Yoshikami D. Omega-conotoxin: direct and persistent blockade of specific types of calcium channels in neurons but not muscle. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4327–4331. doi: 10.1073/pnas.84.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Characteristics of the second inward current in cells isolated from rat ventricular muscle. Proc R Soc Lond B Biol Sci. 1983 Oct 22;219(1217):447–469. doi: 10.1098/rspb.1983.0084. [DOI] [PubMed] [Google Scholar]
- Mitra R., Morad M. Two types of calcium channels in guinea pig ventricular myocytes. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5340–5344. doi: 10.1073/pnas.83.14.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogul D. J., Fox A. P. Evidence for multiple types of Ca2+ channels in acutely isolated hippocampal CA3 neurones of the guinea-pig. J Physiol. 1991 Feb;433:259–281. doi: 10.1113/jphysiol.1991.sp018425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J. B., Tsien R. W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985 Aug 1;316(6027):443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Osaka T., Joyner R. W. Developmental changes in calcium currents of rabbit ventricular cells. Circ Res. 1991 Mar;68(3):788–796. doi: 10.1161/01.res.68.3.788. [DOI] [PubMed] [Google Scholar]
- Richard S., Tiaho F., Charnet P., Nargeot J., Nerbonne J. M. Two pathways for Ca2+ channel gating differentially modulated by physiological stimuli. Am J Physiol. 1990 Jun;258(6 Pt 2):H1872–H1881. doi: 10.1152/ajpheart.1990.258.6.H1872. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Kass R. S. Voltage-dependent block of calcium channel current in the calf cardiac Purkinje fiber by dihydropyridine calcium channel antagonists. Circ Res. 1984 Sep;55(3):336–348. doi: 10.1161/01.res.55.3.336. [DOI] [PubMed] [Google Scholar]
- Tohse N., Nakaya H., Hattori Y., Endou M., Kanno M. Inhibitory effect mediated by alpha 1-adrenoceptors on transient outward current in isolated rat ventricular cells. Pflugers Arch. 1990 Feb;415(5):575–581. doi: 10.1007/BF02583508. [DOI] [PubMed] [Google Scholar]
- Tytgat J., Vereecke J., Carmeliet E. A combined study of sodium current and T-type calcium current in isolated cardiac cells. Pflugers Arch. 1990 Oct;417(2):142–148. doi: 10.1007/BF00370691. [DOI] [PubMed] [Google Scholar]
- Wahler G. M., Rusch N. J., Sperelakis N. 8-Bromo-cyclic GMP inhibits the calcium channel current in embryonic chick ventricular myocytes. Can J Physiol Pharmacol. 1990 Apr;68(4):531–534. doi: 10.1139/y90-076. [DOI] [PubMed] [Google Scholar]


