Abstract
The processing of tyrosinase, which catalyzes the limiting reaction in melanin synthesis, was investigated in melan-p1 melanocytes, which are null at the p locus. Endoglycosidase H digestion showed that a significant fraction of tyrosinase was retained in the endoplasmic reticulum. This retention could be rescued either by transfection of melan-p1 cells with an epitope-tagged wild-type p transcript or by treatment with either bafilomycin A1 or ammonium chloride. We found that the endoplasmic reticulum contains a significant amount of p protein, thus supporting a role for p within this compartment. Using immunofluoresence, we showed that most mature full-length tyrosinase in melan-p1 cells was located in the perinuclear area near the Golgi, in contrast to its punctate melanosomal pattern in wild-type melanocytes. Expression of p in melan-p1 cells restored tyrosinase to melanosomes. Triton X-114 phase separation revealed that an increased amount of tyrosinase was proteolyzed in melan-p1 cells compared with wild-type melanocytes. The proteolyzed tyrosinase was no longer membrane bound, but remained enzymatically active and a large proportion was secreted into the culture medium of melan-p1 cells. We conclude that p regulates posttranslational processing of tyrosinase, and hypopigmentation in melan-p1 cells is the result of altered tyrosinase processing and trafficking.
INTRODUCTION
Tyrosinase (Tyr) catalyzes the rate-limiting reactions in melanin synthesis, converting tyrosine to DOPAquinone. Endoplasmic reticulum (ER) processing of Tyr requires the presence of the chaperone calnexin, which is thought to increase ER retention time for Tyr (Branza-Nichita et al., 1999; Toyofuku et al., 1999). The most common Tyr mutations are associated with ER retention of the protein, presumably the result of misfolding (Berson et al., 2000; Halaban et al., 2000b; Toyofuku et al., 2001). In addition, substrates DOPA and tyrosine promote Tyr exit from the ER and are associated with induction of Tyr activity and melanin synthesis in amelanotic melanoma cells (Halaban et al., 2000a). These studies underline the fact that proper folding and processing of Tyr in the ER are crucial for its subsequent enzymatic activities and melanin deposition. Thus, disruption of melanin synthesis may result not only from mutations at the Tyr locus but also from mutations at loci involved in Tyr processing and transport.
Oculocutaneous albinism type 2 (OCA2), the most common form of albinism worldwide (Ramsay et al., 1992; Durham-Pierre et al., 1994), results from mutations in the pink-eyed dilution gene (p) (Lee et al., 1994). In contrast to oculocutaneous albinism type 1, which results from Tyr mutations, patients with OCA2 express active TYR (King et al., 1985). p was initially thought to be a melanosomal tyrosine transporter (Kugelman and Van Scott, 1968; Rinchik et al., 1993; Lee et al., 1995; Sviderskaya et al., 1997; Rosemblat et al., 1998). However, kinetic studies of tyrosine uptake showed that there was a functional tyrosine transporter system in melanosomes of p-null melanocytes, and no difference in the rate of tyrosine uptake was found between wild-type and p-null melanocytes (Gahl et al., 1995; Potterf et al., 1998). These data suggest that p may not act as a tyrosine transporter in melanosomes.
We have shown recently that p-null melanocytes produce melanin in response to the vacuolar H+-ATPase inhibitors bafilomycin A1 and concanamycin A (Manga and Orlow, 2001). In addition, Fuller et al. (2001) have reported that lightly pigmented Caucasian melanocytes respond to these agents by producing melanin after activation of TYR through a posttranslational mechanism. This increased melanin deposition is associated with alkalinization of the melanosomes as detected with the fluorescent weak base acridine orange. They further showed that optimal Tyr activity occurred at a neutral pH, whereas either pH < 6 or pH > 10 abolishes Tyr activity (Fuller et al., 2001). A new hypothesis has emerged that p may play a role in the alkalinization of melanosomal pH to favor optimal Tyr activity. When p is absent, the pH of melanosomes drops, inactivating Tyr and inhibiting melanin synthesis.
However, other investigators have reached opposite conclusions. Puri et al. (Puri et al., 2000) proposed that the pH of melanosomes in p-null melanocytes is not acidic, whereas an acidic melanosomal environment is important for melanin synthesis (Brilliant, 2001). However, the use of tyrosinase-related protein 1 (Tyrp1), a Tyr family protein that shares 43% amino acid sequence identity with Tyr (Vijayasaradhi et al., 1995), as a melanosomal marker in p-null melanocytes may be inappropriate because Tyrp1, like Tyr, is mislocalized in the absence of p (Manga et al., 2001).
Because p-null melanocytes do not produce melanin, one might predict that they would contain little or no Tyr activity. In fact, extracts of p-null melanocytes exhibit higher levels of both Tyr activity and protein than wild-type melanocytes (Manga et al., 2001). In addition, we and others (Russell, 1949; Moyer, 1966; Rittenhouse, 1968; Rosemblat et al., 1998; Orlow and Brilliant, 1999) have observed defects in melanosomal structure that cannot be easily explained by the hypothesis that p serves as a melanosomal proton pump. We have also shown that the trafficking of melanosomal proteins was altered in p-null melanocytes (Manga et al., 2001), and Tyr is extensively secreted into the culture media after intracellular cleavage. This secretion can be inhibited by incubation with the cysteine protease inhibitor E64 (Manga et al., 2001) as well as by incubation with bafilomycin A1 and concanamycin A (Manga and Orlow, 2001). Mature Tyr protein level increases seven- to eightfold after incubation of p-null melanocytes for 24 h in media containing bafilomycin A1. These data suggest that bafilomycin A1 and concanamycin A induce melanin synthesis in p-null melanocytes by affecting early Tyr processing and trafficking rather than simply by affecting activity at the melanosomal level.
In this study, we explore Tyr processing and trafficking in p-null melanocytes and compare it with that of Tyrp1. We confirm that the trafficking of both Tyr and Tryp1 are altered in p-null melanocytes and show that their misrouting can be corrected by transfection with an expression vector encoding an epitope-tagged wild-type p transcript, or by incubating with bafilomycin A1 or ammonium chloride (NH4Cl). This correction occurs in as early a compartment as the ER where most p is localized, indicating that p plays an important role in controlling the processing of Tyr.
MATERIALS AND METHODS
Cell Culture
Melan-a (a/a, P/P) is an immortalized melanocyte line derived from C57BL/6J mice wild type at the p locus. Melan-p1 is an immortalized melanocyte line from mice lacking p gene transcripts due to overlapping deletions (Sviderskaya et al., 1997) (a/a, pcp/p25H). Melan-c is an immortalized melanocyte homozygous for a point mutation (C85S) at the Tyr locus (Bennett et al., 1989). The melan-p1+p cell line is a clone of melan-p1 cells stably transfected with an expression plasmid encoding an epitope-tagged wild-type p transcript (p-his-v5) (Manga and Orlow, 2001). COS-1 cells were obtained from American Type Culture Collection (Mannasas, VA). All cell lines were maintained in RPMI medium (Sigma-Aldrich, St. Louis, MO) as described previously (Manga and Orlow, 2001). The media for melan-c cells were supplemented with 0.007 μl/ml β-mercaptoethanol. Bafilomycin A1 (Wako, Richmond, VA) and NH4Cl (Fisher Scientific, Pittsburgh, PA) were added to final concentrations of 50 mM and 10 mM for 24 h.
Antibodies
αPep7 and αPep1 antisera were raised against the C terminus of mouse Tyr and Tyrp1, respectively (Genemed, South San Francisco, CA) based on antigenic peptide sequences from Dr. V. Hearing (Jimenez et al., 1991). For immunofluoresence analysis, αPep7 was further affinity purified using the Pep7 peptide linked to an Affi-gel15 column (Bio-Rad, Hercules, CA). The polyclonal antibody αPep13 was generated against the C terminus of the mouse silver protein (Zhou et al., 1994). The following antibodies were also used: GRP78/bip, syntaxin 6, and Vti1b (Transduction Laboratories, Lexington, KY); ribophorin (obtained from Dr. G. Kreibich, New York University Medical Center, New York, NY); anti-V5 antibody (Invitrogen, San Diego, CA); Mel-5 (Signet, Dedham, MA), and Calnexin (StressGen, Victoria, British Columbia, Canada).
Cell Extract
Cells were harvested in extraction buffer (1% Triton X-100, 50 mM Tris, 2 mM EDTA, 150 mM NaCl, pH 7.5) containing protease inhibitor cocktail (Roche Applied Science, Mannheim, Germany) after two washes with ice-cold phosphate-buffered saline (PBS). The lysates were centrifuged at 10,000 × g, 4°C for 10 min in a microcentrifuge. The protein concentrations of supernatants were measured with a protein assay kit (Bio-Rad) and normalized to 2 mg/ml.
Small Granule Fraction (SGF) and Large Granule Fraction (LGF) Preparation
Cells were rinsed three times with ice-cold PBS, scraped with 0.25 M sucrose in homogenization buffer (10 mM Tris-HCl, 10 mM KCl, pH 7.5) containing protease inhibitor cocktail. Cells were homogenized using a ball-bearing homogenizer with 30 strokes then centrifuged at 100 × g for 10 min to remove cell debris. The supernatants were normalized to 2 mg/ml and centrifuged at 10,000 × g, 4°C for 15 min, to collect LGF. The resulting supernatants were again centrifuged at 100,000 × g, 4°C for 1 h, to collect the SGF. Alternatively, after removal of cell debris, the initial supernatants were centrifuged at 100,000 × g, 4°C for 1 h, to collect total granule fraction.
Sucrose Gradient Centrifugation and Percoll Gradient Centrifugation
The total granule fraction was resuspended in 0.5 ml of 0.25 M sucrose and layered on a stepwise sucrose gradient. The gradients consisted of 0.5-ml steps in increments of 0.2 M from 0.8 to 2.0 M. Sucrose gradients were centrifuged in a SW60 rotor at 100,000 × g, 4°C for 1 h. Fractions were collected with a pipette from top to bottom.
For Percoll gradient fractionation, cells were homogenized as described above in the presence of Percoll homogenization buffer (10 mM HEPES, 1 mM EDTA, 0.25 M sucrose, pH 7.2) containing protease inhibitor cocktail. The supernatants were adjusted to 0.86 mg/ml protein concentration after a 100-g spin, and mixed with 90% Percoll (Sigma-Aldrich) in Percoll homogenization buffer to a final concentration of 31.5% Percoll. The mixture was centrifuged in a Vti65.2 (Beckman-Coulter, Fullerton, CA) at 18,000 rpm, 4°C for 48 min. Fractions were collected using a peristaltic pump from bottom to top. Each fraction was further centrifuged at 100,000 × g, 4°C for 30 min, to remove Percoll.
Western Blot Analysis
Proteins were separated by 7% SDS-PAGE and transferred onto membranes (Immobilon-P; Millipore, Waltham, MA)
Immunofluorescence Microscopy
Cells were grown on coverslips (Fisher Scientific) for 48 h, washed three times with ice-cold PBS, and fixed with −20°C methanol for 5 min and processed as described previously (Shen et al., 2001). The slides were analyzed using either a confocal microscope (LSM510; Carl Zeiss, Thornwood, NY) or a digital fluorescence microscope (Axiophot; Carl Zeiss). All data were analyzed with 100× oil lens and processed with Adobe Photoshop 6.0 (Adobe Systems, Mountain View, CA).
Triton X-114 Phase Separation
The SGF and LGF were resuspended in 600 μl of 0.25 M sucrose. Each suspension was divided into two 300-μl portions. Portion 1 was kept without phase separation and labeled as total phase. Triton X-114 was added to portion 2 to a final concentration of 1%. The mixture was incubated at 37°C for 5 min and then centrifuged at 10,000 × g for 5 min. The upper aqueous phase was separated from the lower detergent phase. Triton X-114 was added to separated aqueous phase to a final concentration of 1% and the extraction was repeated two more times. The resulting aqueous phase was brought to 300 μl by using 0.25 M sucrose. The lower detergent phases were combined and brought to 300 μl with 0.25 M sucrose. The insoluble pellet resulting from LGF phase separation, present below the detergent phase, was separated and resuspended in 300 μl of 0.25 M sucrose. Thirty microliters of each phase were used in Western blotting or Tyr DOPA oxidase assays.
Tyrosinase Activity
The tyrosine hydroxylase activity of Tyr was determined radiometrically, in either duplicate or triplicate, as described previously with modification (Pomerantz, 1969; Manga and Orlow, 2001).
DOPA Staining on Nondenaturing SDS-PAGE
DOPA oxidase activity of Tyr was performed as reported previously (Jimenez-Cervantes et al., 1993) by staining samples with DOPA and 3-methyl-2-benzothiazolinone hydrozone (MBTH; Sigma-Aldrich) on nondenaturing polyacrylamide gels.
Glycosidase Analysis
Glycosidase digestion was based on the protocol from New England Biolabs (Beverly, MA). Twenty micrograms of protein extract was denatured in 0.5% SDS, 1% β-mercaptoethanol at 100°C for 10 min. Then 1/10 volumes of each 0.5 M sodium phosphate (pH 7.5) and 10% NP-40 were added. Two (500 U/μl) N-glycosidase F (New England Biolabs) or (5 mU/μl) endoglycosidase H (Roche Applied Science) was used for digestion at 37°C for 1 h. The reaction was terminated with SDS sample loading buffer and boiled at 100°C for 2 min.
RESULTS
A Higher Percentage of Tyr Is Retained in ER of Melan-p1 Cells Than in Wild-Type Cells
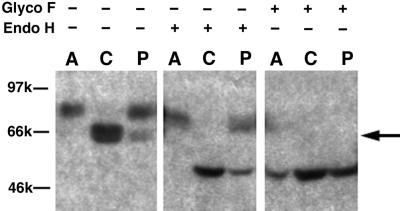
In addition to the 70-kDa mature form present in melan-a cells, a 66-kDa form of Tyr was routinely detected upon Western blotting of melan-p1 cell lysates with αPep7, which recognizes the C terminus of Tyr (Figure 1). Densitometric analysis revealed that approximately one-third of Tyr in melan-p1 cells is in this form. This form of Tyr was found to comigrate with Tyr from melan-c cells, derived from a mouse homozygous for point mutations in Tyr, which results in ER retention of Tyr (Halaban et al., 2000b).
Figure 1.
Detection of an ER-retained form of Tyr in melan-p1 cells. Postnuclear supernatants (PNS) from melan-a, melan-c, and melan-p1 cells were probed by immunoblotting with an antibody against the C terminus of Tyr, (αPep7). Lanes 1–3 are PNS from melan-a cells, melan-c cells, and melan-p1 cells. Lanes 4–6 are PNS treated with endoglycosidase H. Lanes 7–9 are PNS treated with N-glycosidase F. The ER-retained Tyr band is indicated with an arrow. A, melan-a cells; C, melan-c cells; and p, melan-p1 cells.
When cell extracts were treated with endoglycosidase H, the 66-kDa form of Tyr shifted to 56 kDa, approximately equal to the molecular weight of unglycosylated mouse Tyr. Endoglycosidase H removes high-mannose glycans from glycoproteins in the ER. Complex glycan processing occurs in the medial-Golgi and renders proteins resistant to endoglycosidase H digestion (Halaban et al., 1997). Thus, this 66-kDa form represents an ER-retained Tyr.
The mature 70-kDa form of Tyr showed a slight shift to a more rapidly migrating band upon endoglycosidase H treatment (lane 1 vs. 4). Mouse Tyr has been shown to use four of the six potential glycosylation sites (Branza-Nichita et al., 2000). This small shift was likely the result of a lack high-mannose glycan processing due to inability of accessing this glycan site by Golgi enzymes.
N-Glycosidase F removes all forms of glycans. When cell extracts were treated with N-glycosidase F, both mature Tyr and ER-retained Tyr migrated as 56-kDa bands, indicating that the difference between mature and ER-retained forms of Tyr was their glycosylation pattern, and not the amino acid length of the Tyr.
Stable Transfectant Melan-p1+p Exhibits More Efficient ER Processing of Tyr
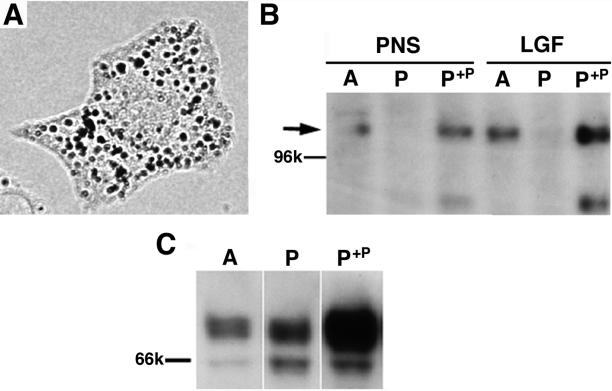
To test whether ER retention was the direct result of the absence of p, we studied the processing of Tyr in a melan-p1 cell line stably transfected to express an epitope-tagged p transcript (melan-p1+p) (Manga and Orlow, 2001). An expression plasmid was constructed using polymerase chain reaction amplification of the p gene cDNA, followed by TOPO cloning (Invitrogen) into the pcDNA3.1 vector. The resulting plasmid generates a fusion product consisting of the entire coding region of the p gene tagged at the C terminus with the 6-His and v5 epitopes. This vector was transfected into melan-p1 cells by using Fugene-6 (Roche Applied Science), and a stable clone was selected by using 800 μg/ml active G418 (Sigma-Aldrich). This clone was selected based on melanin production when cultured in media with low concentrations of tyrosine. The resulting melan-p1+p clone produces melanin and expresses p (Figure 2, A and B).
Figure 2.
Expression of p is accompanied by more efficient ER processing of Tyr in melan-p1+p. Melan-p1+p cells are melan-p1 cells stably transfected with a p-his-v5 vector. (A) Melan-p1+p cells were dark and produced mature melanosomes. (B) Antibody against p detected the 110-kDa protein (arrow) in melan-a and melan-p1+p cells, but not in melan-p1 cells in both PNS and LGF, which is enriched for ER and melanosomes. (C) LGF of melan-a, melan-p1, and melan-p1+p cells was probed with αPep7. p+p, melan-p1+p.
The relative ratio between ER-retained Tyr and mature Tyr in melan-p1+p cells was similar to that in melan-a cells and was lower than that in melan-p1 cells (Figure 2C). The amount of mature Tyr in melan-p1+p is much higher than that of melan-p1 cells, thus indicating that maturation of Tyr is enhanced by expression of p.
Full-Length Tyr in Melan-p1 Cell Is Predominantly Located near Golgi; Expression of p Restores Tyr to Melanosomes
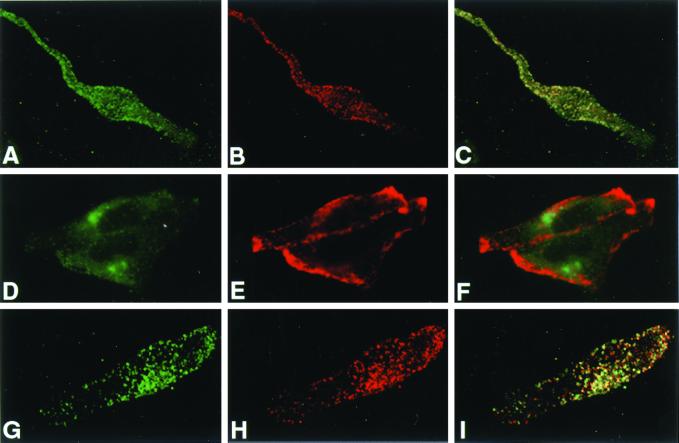
We and others have shown previously that the distribution of Tyr is altered in melan-p1 cells (Potterf et al., 1998; Manga et al., 2001). As shown in Figure 3, Tyr in melan-a cells exhibited a punctate staining pattern, typical of melanosomal localization. In contrast, in melan-p1 cells there was little punctate staining. Instead, αPep7 detected strong staining in the perinuclear area, close to the location of the Golgi in these cells (Figure 4). This staining partially overlapped with Golgi marker VTI1b (Figure 4, D–F) and consisted of multiple punctate vesicles (Figures 3, 4, and 6B). Because αPep7 detects the C terminus of Tyr, Tyr seems to be cleaved in this organelle (most likely trans-Golgi network) or in a compartment immediately distal to this organelle. Melan-p1+p showed a similar punctate Tyr staining pattern to that observed in melan-a cells. This confirmed that the altered Tyr trafficking in melan-p1 cells was the direct result of the absence of p.
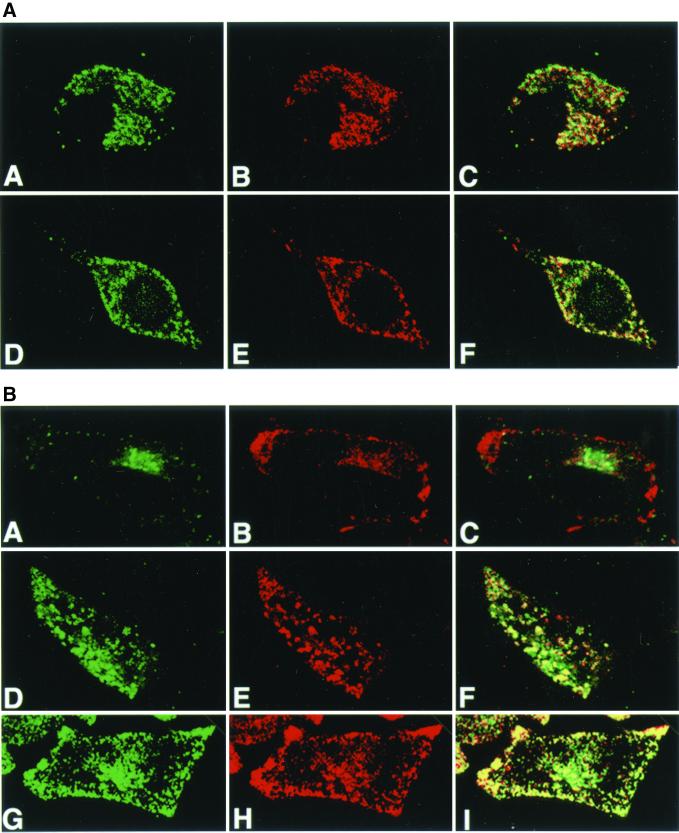
Figure 3.
Trafficking of both Tyr and Tyrp1 is altered in melan-p1 cells. Immunofluorescence analysis of melan-a cells (A–C), melan-p1 cells (D–F), and melan-p1+p (G–I) stained with αPep7 (A, D, and G) and Mel-5 (B, E, and H). C, F, and I are the merged images. αPep7 detects the C terminus of Tyr and Mel-5 detects a lumenal epitope of Tyrp1.
Figure 4.
Tyr in melan-p1 cells exhibits a perinuclear location near the Golgi. Immunofluorescence analysis of melan-a cells (A–C), melan-p1 cells (D–F), and melan-p1+p (G–I) stained with αPep7 (Tyr) (A, D, and G) and VTI1b (Golgi) (B, E, and H). C, F, and I are merged images.
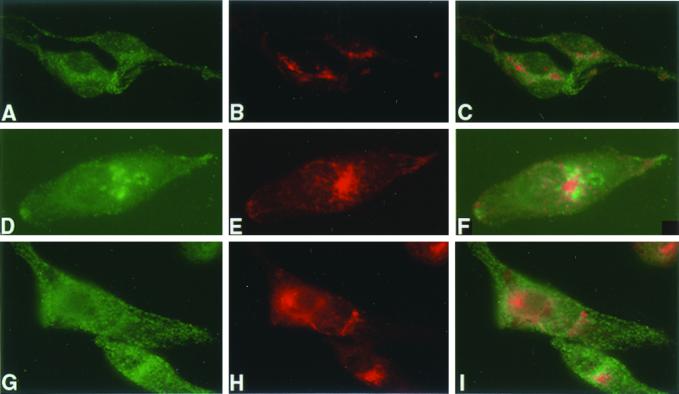
Figure 6.
Tyr trafficking in melan-p1 cell is restored by bafilomycin A1 and NH4Cl. (A) Confocal immunofluoresence analysis of melan-a cells with αPep7 (A and D) and Mel-5 (B and E). C and F are merged images. A–C, melan-a cells with 50 nM bafilomycin A1; D–F, melan-a cells with 10 mM NH4Cl. (B) Confocal immunofluorescence analysis of melan-p1 cells with αPep7 (A, D, and G) and Mel-5 (B, E, and H). C, F, and I are merged pictures. A–C, melan-p1 cells; D–F, melan-p1 cell with 50 nM bafilomycin A1; G–I, melan-p1 cell with 10 mM NH4Cl.
The distribution of Tyrp1 is also altered by the absence of p (Manga et al., 2001) (Figure 4). Although some punctate staining was found within melan-p1 cells, Tyrp1 staining was most intense near the cell membrane (Figure 3E), but not on the cell membrane (our unpublished data). This peripheral distribution pattern was also corrected in melan-p1+p cells and resembled that seen in melan-a cells. Immunofluorescence studies of Tyrp1 localization analysis with Mel5 and αPep1 showed nearly perfect overlap in each cell type (our unpublished data). Because Mel5 detects an epitope in the lumenal domain of Tyrp1 and αPep1 detects the cytosolic carboxyl-terminus of Tyrp1, Tyrp1 does not seem to be cleaved in melan-p1 cells.
Bafilomycin A1 and NH4Cl Induce Melanin Synthesis in Melan-p1 Cells by Promoting ER Processing of Tyr, Preventing Tyr Degradation, and Restoring Proper Trafficking of Tyr and Tyrp1
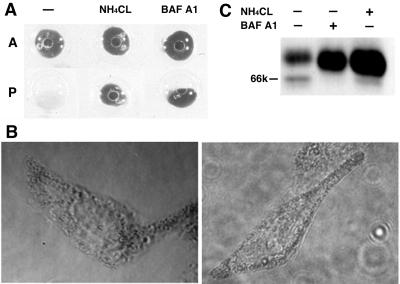
Treatment of melan-p1 cells with bafilomycin A1 and concanamycin A can induce melanin production (Manga and Orlow, 2001; Figure 5, A and B). Figure 5C shows that in part, this melanin synthesis is the result of more efficient ER processing of Tyr. Melan-p1 cells become melanized when treated with 50 nM bafilomycin A1 (Figure 5A). Under these conditions, the intensity of the 66-kDa ER-retained Tyr diminished and that of the 70-kDa mature form increased by seven- to eightfold (Figure 5C).
Figure 5.
Bafilomycin A1 and NH4Cl induce melanin synthesis, reduce Tyr retention in the ER, and increase Tyr maturation in melan-p1 cells. (A) Cells were treated with 50 nM bafilomycin A1 or 10 mM NH4Cl for 24 h then harvested in extraction buffer and centrifuged at 10,000 × g, 4°C for 10 min. A, melan-a; p, melan-p1. (B) Dark melanosomes are produced in melan-p1 cells treated with bafilomycin A1 (left) and NH4Cl (right). (C) Western blotting with αPep7 of cell extract of melan-p1 cells treated with bafilomycin A1 and NH4Cl.
NH4Cl can also induce melanin production in melan-p1 cells. At 10 mM NH4Cl, melan-p1 cells become melanized (Figure 5) and, as with bafilomycin A1, the ER-retained Tyr was reduced (although to a lesser degree than with 50 nM bafilomycin A1), and the amount of 70-kDa mature Tyr protein level increased by seven- to eightfold.
In addition to melanin production and more efficient ER processing of Tyr in the presence of bafilomycin A1 and NH4Cl, there is a restoration of Tyr and Tyrp1 trafficking to melanosomes in melan-p1 cells treated with those agents as seen in Figure 6B. Neither bafilomycin A1 nor NH4Cl affect their trafficking in melan-a cells (Figure 6A). This indicates that Tyr stability and its trafficking to melanosomes are directly linked to the ER processing.
The Majority of p Is Located in the ER
ER retention of Tyr in melan-p1 cells and correction of ER processing by transfection with a p expression vector or by treatment with bafilomycin A1 and NH4Cl all suggest that p plays a role in controlling posttranslational modification of Tyr in the ER. This prompted us to reexamine the location of p. It was generally thought that p was located in melanosomes (Rosemblat et al., 1994; Donatien and Orlow, 1995). However, a combination of data from sucrose gradient and Percoll gradient fractionation as well as immunofluorescence microscopy reveals that the ER contains a significant amount of p protein.
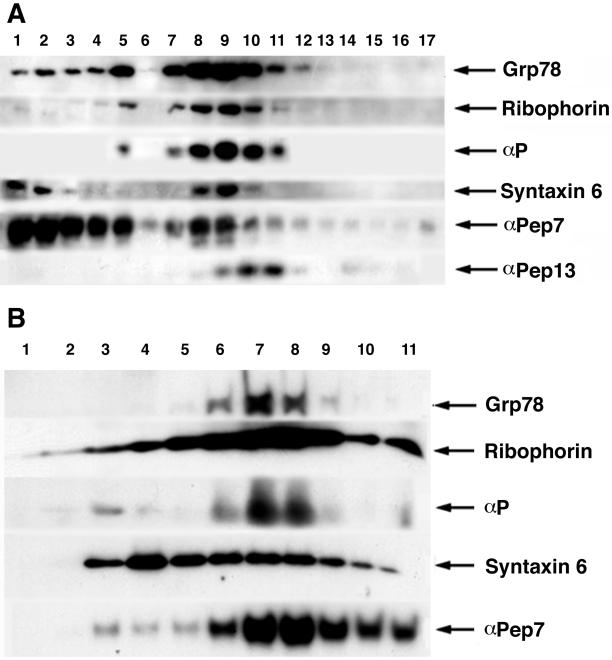
Figure 7A shows the results of Percoll gradient fractionation of melan-a cells. Percoll gradient fractionation gave excellent separation between mature melanosomes and other organelles such as ER and Golgi. Melanosomes were located in the densest fractions, at the bottom of the gradient from fraction 1 to 3. This was confirmed by observing melanin deposition in these fractions (our unpublished data). The ER markers ribophorin and αGrp78/bip exhibited two peaks, a major one from fractions 8 to 10 and a minor one at fraction 5. Similarly, p has a biphasic distribution overlapping perfectly with both ER markers but not with melanosomal markers. The Golgi marker syntaxin 6 showed a similar distribution to ER markers in the Percoll gradient and partially overlapped with p. In addition, it was also present in fractions 1 and 2. Tyr distribution was biphasic with a predominant peak in the melanosome fractions 1–3 and a minor peak in the Golgi/ER fractions. To determine whether p is located in premelanosomes, we used αPep13 against the silver protein as a premelanosome marker (Zhou et al., 1994; Raposo et al., 2001). The premelanosomes were found between fractions 9 and 11, in a single peak and one fraction lighter than p. Thus, most p is not present in premelanosomes.
Figure 7.
ER contains a significant amount of p protein. (A) Percoll gradient of PNS from melan-a cells. The dense fractions are on the left and light fractions are on the right. Immunoblotting was performed with ER marker Grp78/bip, ribophorin, p antibody, Golgi marker syntaxin 6, αPep7, and αPep13. (B) Sucrose gradient analysis of total granule fractions from melan-a cells. The lighter fractions are on the left and dense fractions are on the right. Immunoblotting was performed with ER markers Grp78/bip, ribophorin, p antibody, syntaxin 6, and αPep7.
To further distinguish ER from Golgi, we performed sucrose gradient fractionation, which gave good separation between Golgi and ER/melanosomes; however, ER and melanosomes could not be well separated from each other. Figure 7B shows the results of sucrose gradient fractionation of melan-a cells. The distribution of the ER markers grp78/bip and ribophorin overlapped with that of p. The Golgi marker syntaxin 6 was distributed more broadly with a peak at fraction 4. The peak of Tyr distribution was at the same location as in ER fractions 7 and 8. When the sucrose gradient and Percoll gradient data are considered together, they indicate that the ER of melan-a cells contains a significant amount of p protein.
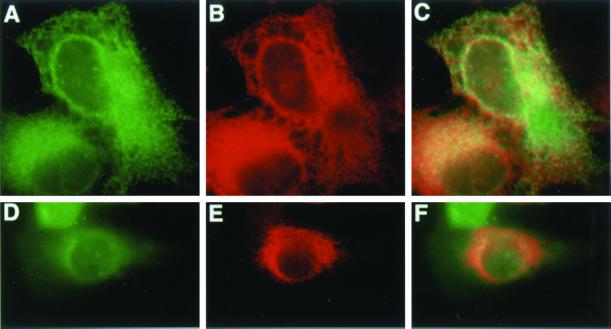
To further study p subcellular localization, we transfected the epitope-tagged p expression plasmid into COS-1 and melan-a cells. Figure 8 shows that p colocalizes with the ER marker ribophorin and calnexin after transient transfection in both cell types.
Figure 8.
Transiently transfected p is detected in the ER in COS-1 and melan-a cells. P-his-v5 vector is transfected to COS-1 (A–C) and melan-a cells (D–F) for 48 h and detected with V5 antibody (A and D) and ER markers ribophorin (B) and calnexin (E). C and F are merged pictures.
Tyr Is Extensively Cleaved in Melan-p1 Cells
Our previous data showed high levels of Tyr activity in the culture medium of melan-p1 cells. This Tyr secretion was reduced by incubation with E64, a cysteine protease inhibitor. We thus hypothesized that Tyr was cleaved in melan-p1 cells and subsequently secreted into the culture medium (Manga et al., 2001). Triton X-114 phase separation was used to characterize the cleavage pattern of Tyr. If Tyr is cleaved in its lumenal domain, the cleaved Tyr should fall into the lumen and separate into the aqueous phases, and the intact Tyr being a type 1 membrane protein should separate into detergent phases.
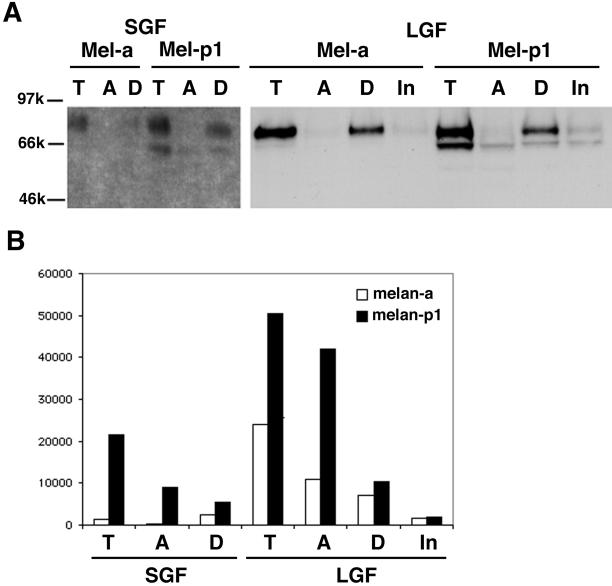
In Figure 9, SGF and LGF were subjected to Triton X-114 phase separation. SGF has been shown to be enriched for small vesicles and cell membrane fragments, whereas LGF contains predominantly ER, melanosomes, and mitochondria (Seiji et al., 1963). Western blotting with αPep7 showed full-length Tyr protein was distributed exclusively in the detergent phase in both SGF and LGF (Figure 9A). The full-length Tyr protein was not detected in the aqueous phase or in the insoluble phase. However, most Tyr activity was detected in the aqueous phase for both SGF and LGF of melan-a and melan-p1 cells (Figure 9B). Tyr activity in the aqueous phase must be the result of a cleaved form of Tyr protein, not detectable with αPep7, which only recognizes Tyr cytosolic tail.
Figure 9.
Most Tyr activity is found in the aqueous phase, whereas the full-length Tyr is only in detergent phase after Triton X-114 separation. SGF and LGF from melan-a and melan-p cells are phase separated with Triton X-114. T, before phase separation; A, aqueous phase; D, detergent phase; In, insoluble phase. (A) Western blot with αPep7. (B) Tyrosine hydroxylase activity of Tyr on the separated phases.
We confirm the observation (Manga et al., 2001) that the SGF of melan-p1 cells contains abundant Tyr activity, whereas the SGF of melan-a cells does not. In addition, the ratio of Tyr activity in the aqueous phase to that in the detergent phase in the LGF of melan-p1 cells (4:1) was much higher than that in melan-a cells (1.4:1). These data indicate that Tyr cleavage may be part of the normal turnover process in melan-a cells, but occurs at a much higher rate in the absence of p.
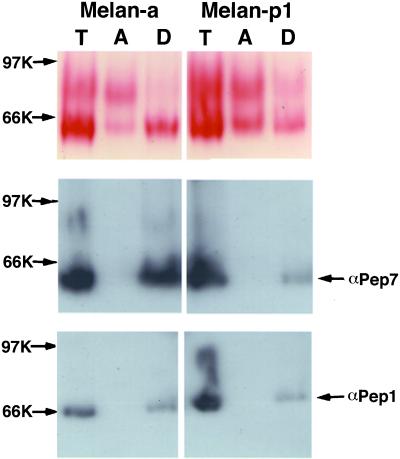
Staining for the DOPA oxidase activity of Tyr performed on native electrophoresis gels confirmed at least two distinguishable products in the aqueous phase in both melan-a and melan-p1 cells (Figure 10). The location of the full-length Tyr in nondenaturing gels was determined with αPep7. DOPA staining detected a single band in the detergent phase corresponding to the location of full-length Tyr. The aqueous phase contained one band that migrated slightly slower than the full-length Tyr and another band that ran much more slowly. The intensity of the bands in the aqueous phase of melan-p1 cells was stronger than that of melan-a cells, confirming increased cleavage in melan-p1 cells.
Figure 10.
Staining of DOPA oxidase activity of Tyr shows increased cleavage rate for Tyr in melan-p1 cells. PNS from melan-a and melan-p cells were phase separated with Triton X-114. Equal amounts of protein were loaded onto nondenaturing gels. The top panel shows DOPA staining. The locations of full-length Tyr and Tyrp1 in the gel are determined by Western blotting with αPep7 and αPep1.
Because some data (Jimenez-Cervantes et al., 1993; Negroiu et al., 1999) suggest that Tyrp1 may partially contribute to the DOPA oxidase activity in melanocytes, αPep1 was used to locate Tyrp1 (Figure 10). Tyrp1 separated exclusively into the detergent phase. The DOPA-positive band in the detergent phase corresponded to the location of Tyr, not to that of Tyrp1. Thus, only Tyr DOPA oxidase activity was detectable in this assay.
DISCUSSION
This study explored Tyr processing and trafficking in melan-p1 cells. The enzymatic activities of Tyr and its localization to melanosomes are essential for pigment deposition. Most Tyr mutations examined to date are associated with ER retention, rather than loss of Tyr activity as was predicted previously. The temperature-sensitive Tyr mutant R402Q can exit the ER at 31°C, but accumulates in the ER at 37°C (Berson et al., 2000). Thus, proper processing of Tyr in the ER is vital to its enzymatic activities and subcellular localization.
Our previous sucrose gradient and electron microscopy studies and works of others have shown that the distribution of Tyr protein and enzymatic activity was altered in melan-p1 cells, which lack p (Potterf et al., 1998; Manga et al., 2001). Herein, we further explore this mislocalization and show that it originates in the ER. Melan-a cells exhibit more efficient Tyr processing in the ER than do melan-p1 cells. To confirm that any observations made were the direct result of the lack of p by melan-p1 cells, we corrected the defect in melan-p1 cells by stable transfection of an epitope-tagged p expression plasmid. The phenotype of the resulting melan-p1+p cells was similar to that of melan-a cells with increased melanin deposition, enhanced ER processing of Tyr, and distribution of Tyr to melanosomes.
The distribution of Tyr is dramatically altered in melan-p1 cells. Tyr colocalizes with Tyrp1 in melan-a and melan-p1+p cells, whereas there is little overlap in melan-p1 cells. Tyr shows a strong perinuclear staining pattern near the Golgi in melan-p1 cells, whereas Tyrp1 was seen at the periphery of the cells. Their distributions in melan-p1 cells are remarkably similar to that observed in the glycosphingolipid-deficient GM95 mouse melanoma cell line, in which Tyr is predominantly located near the Golgi and Tyrp1 reaches peripheral vacuoles through cell membrane (Sprong et al., 2001). Thus, it raises the possibility that p may play a role in glycosphingolipid synthesis or traffic. The lack of reactivity of melanosomes in melan-p1 cells with αPep7, which recognizes the C terminus of Tyr, implies that full-length Tyr is not present in melanosomes. Together with previous data showing Tyr secretion into the medium by melan-p1 cells, and the observation that secretion is prevented by incubation with the cysteine protease inhibitor E64 (Manga et al., 2001), the full-length Tyr seems to be cleaved at or immediately after exiting this perinuclear organelle. The resulting truncated Tyr lacks melanosomal trafficking signals, with resultant secretion via the default trafficking pathway. This hypothesis is further strengthened by the observation that there is an increased Tyr cleavage rate in melan-p1 cells, as shown by Triton X-114 phase separation and DOPA oxidase staining of nondenaturing gels.
The effects of bafilomycin A1 and NH4Cl on melanin synthesis of melan-p1 cells seem to be twofold. They promote the ER processing of Tyr and reduce the amount of Tyr trapped in the ER. In addition, they prevent the degradation of mature Tyr before it reaches melanosomes. Their effects on the activity of already processed Tyr do not seem to be rate-limiting steps for melanin synthesis in melan-p1 cells. Bafilomycin A1 (50 nM) actually decreased Tyr activity by half and 10 mM NH4Cl increased Tyr activity by 30% as assessed in extracts of treated cells (our unpublished data). Bafilomycin A1 and NH4Cl are known to raise the pH within organelles. Ishidoh et al. (1999) reported that bafilomycin A1, which can increase lysosomal pH, causes the degradation of cathepsin B, D, and L. We reported that the cathepsin family inhibitor E64 can prevent Tyr secretion into the medium by melan-p1 cells (Manga et al., 2001). Thus, the effects of bafilomycin A1 on Tyr degradation in melan-p1 may be mediated through a reduction in cathepsin proteases. High tyrosine can also induce melan-p1 cells to produce melanin (Sviderskaya et al., 1997; Rosemblat et al., 1998). This is associated with an increase in the concentration of mature Tyr. However, the amount of Tyr secreted into culture medium dropped only slightly (Manga et al., 2001). Thus, the effect of tyrosine on the stability of Tyr seems to be different from those of bafilomycin A1 and NH4Cl. Tyrosine may act in the ER to promote correct folding of Tyr (Halaban et al., 2000a) rather than in preventing Tyr degradation.
The subcellular location of p was explored further in this study. Using Percoll and sucrose gradient fractionation in combination with immunofluorescence microscopy, we show that the ER contains a significant amount of p protein. This is in contrast to our previous prediction (Rosemblat et al., 1994) that p is a melanosomal protein. This prediction was based in part on the colocalization of p with mutated Tyr in melan-c cells, which was assumed at the time to be situated in melanosomes, but to be enzymatically inactive. It has since been shown that the mutated Tyr in melan-c cells is in fact trapped in the ER and subsequently degraded (Halaban et al., 2000b). Indeed, the colocalization of Tyr and p in melan-c cells further strengthens our conclusions regarding the ER localization of p. However, it is still possible that a portion of total p may locate in other organelles. Previous experiments have shown that increasing melanin deposition is inversely proportional to p detection by Western blotting (Donatien and Orlow, 1995). Thus, mature melanosomes may still contain some p, which is not detectable by the techniques used in the current study.
The function of p is not well understood. Hypotheses regarding p function include the following: 1) p transports tyrosine into melanosomes (Lee et al., 1995). 2) p plays a structural role in melanosomes (Donatien and Orlow, 1995; Rosemblat et al., 1998) or is involved in melanosome biogenesis (Orlow and Brilliant, 1999). 3) p plays a role as a pH regulator in melanosomes and provides an optimal pH for Tyr activity (Puri et al., 2000; Brilliant, 2001). These hypotheses are based on the assumption that p functions in melanosomes. Our data show that p in fact controls very early steps in the melanogenic pathway.
ER processing of Tyr, its intracellular cleavage, trafficking to melanosomes, and secretion into culture medium are tightly linked. For example, bafilomycin A1-treated melan-p1 cells, like melan-a cells, have more efficient ER processing of Tyr, a reduction of intracellular cleavage, restoration of Tyr trafficking to the melanosome, and diminished secretion into the culture media. We thus propose the following model for p action. p facilitates the creation of optimal folding conditions for Tyr in the ER. It may aid in alkalinization of pH in the ER or it may be a transporter of tyrosine or thiols into the ER. Alternatively, it may play a role in generating or regulating the intracellular movement of glycosphingolipids, which are important for melanosomal proteins trafficking (Sprong et al., 2001). The loss of this optimal folding condition in melan-p1 cells results in increased Tyr trapping in the ER and misfolding of Tyr into a protease sensitive form. The misfolded Tyr can exit ER and is functional. However, it is cleaved at a higher rate after exiting the Golgi and is secreted into the culture medium instead of trafficking to melanosomes. Optimal folding conditions in melan-p1 cells can be restored by bafilomycin A1 and high tyrosine. By increasing the pH of organelles, NH4Cl and bafilomycin A1 can also block the degradation of the protease sensitive Tyr in melan-p1 cells. How p could generate this optimal folding condition remains to be determined. Based on its structure, it is indeed likely to be a transporter of some kind. Whatever its function, p is clearly crucial for posttranslational processing of Tyr.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants EY-10223 and AR-41880 (to S.J.O.) and by funding from the Anaderm Research Corporation.
Abbreviations used:
- ER
endoplasmic reticulum
- LGF
large granule fraction
- OCA2
oculocutaneous albinism type 2
- p
mouse pink-eyed dilution protein
- SGF
small granule fraction
- Tyr
mouse tyrosinase
- TYR
human tyrosinase
- Tyrp1
mouse tyrosinase related protein 1
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.02–02–0022. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.02–02–0022.
REFERENCES
- Bennett DC, Cooper PJ, Dexter TJ, Devlin LM, Heasman J, Nester B. Cloned mouse melanocyte lines carrying the germline mutations albino and brown: complementation in culture. Development. 1989;105:379–385. doi: 10.1242/dev.105.2.379. [DOI] [PubMed] [Google Scholar]
- Berson JF, Frank DW, Calvo PA, Bieler BM, Marks MS. A common temperature-sensitive allelic form of human tyrosinase is retained in the endoplasmic reticulum at the nonpermissive temperature. J Biol Chem. 2000;275:12281–12289. doi: 10.1074/jbc.275.16.12281. [DOI] [PubMed] [Google Scholar]
- Branza-Nichita N, Negroiu G, Petrescu AJ, Garman EF, Platt FM, Wormald MR, Dwek RA, Petrescu SM. Mutations at critical N-glycosylation sites reduce tyrosinase activity by altering folding and quality control. J Biol Chem. 2000;275:8169–8175. doi: 10.1074/jbc.275.11.8169. [DOI] [PubMed] [Google Scholar]
- Branza-Nichita N, Petrescu AJ, Dwek RA, Wormald MR, Platt FM, Petrescu SM. Tyrosinase folding and copper loading in vivo: a crucial role for calnexin and alpha-glucosidase II. Biochem Biophys Res Commun. 1999;261:720–725. doi: 10.1006/bbrc.1999.1030. [DOI] [PubMed] [Google Scholar]
- Brilliant MH. The mouse p (pink-eyed dilution) and human P genes, oculocutaneous albinism type 2 (OCA2), and melanosomal pH. Pigment Cell Res. 2001;14:86–93. doi: 10.1034/j.1600-0749.2001.140203.x. [DOI] [PubMed] [Google Scholar]
- Donatien PD, Orlow SJ. Interaction of melanosomal proteins with melanin. Eur J Biochem. 1995;232:159–164. doi: 10.1111/j.1432-1033.1995.tb20794.x. [DOI] [PubMed] [Google Scholar]
- Durham-Pierre D, Gardner JM, Nakatsu Y, King RA, Francke U, Ching A, Aquaron R, del Marmol V, Brilliant MH. African origin of an intragenic deletion of the human P gene in tyrosinase positive oculocutaneous albinism. Nat Genet. 1994;7:176–179. doi: 10.1038/ng0694-176. [DOI] [PubMed] [Google Scholar]
- Fuller BB, Spaulding DT, Smith DR. Regulation of the catalytic activity of preexisting tyrosinase in black and Caucasian human melanocyte cell cultures, Exp. Cell Res. 2001;262:197–208. doi: 10.1006/excr.2000.5092. [DOI] [PubMed] [Google Scholar]
- Gahl WA, Potterf B, Durhampierre D, Brilliant MH, Hearing VJ. Melanosomal tyrosine transport in normal and pink-eyed dilution murine melanocytes. Pigment Cell Res. 1995;8:229–233. doi: 10.1111/j.1600-0749.1995.tb00668.x. [DOI] [PubMed] [Google Scholar]
- Halaban R, Cheng E, Svedine S, Aron R, Hebert DN. Proper folding, and ER to Golgi transport of tyrosinase are induced by its substrates, DOPA, and tyrosine. J Biol Chem. 2000a;20:20. doi: 10.1074/jbc.M008703200. [DOI] [PubMed] [Google Scholar]
- Halaban R, Cheng E, Zhang Y, Moellmann G, Hanlon D, Michalak M, Setaluri V, Hebert DN. Aberrant retention of tyrosinase in the endoplasmic reticulum mediates accelerated degradation of the enzyme and contributes to the dedifferentiated phenotype of amelanotic melanoma cells. Proc Natl Acad Sci USA. 1997;94:6210–6215. doi: 10.1073/pnas.94.12.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaban R, Svedine S, Cheng E, Smicun Y, Aron R, Hebert DN. Endoplasmic reticulum retention is a common defect associated with tyrosinase-negative albinism. Proc Natl Acad Sci USA. 2000b;97:5889–5894. doi: 10.1073/pnas.97.11.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishidoh K, Takeda-Ezaki M, Watanabe S, Sato N, Aihara M, Imagawa K, Kikuchi M, Kominami E. Analysis of where and which types of proteinases participate in lysosomal proteinase processing using bafilomycin A1 and Helicobacter pylori Vac A toxin. J Biochem. 1999;125:770–779. doi: 10.1093/oxfordjournals.jbchem.a022348. [DOI] [PubMed] [Google Scholar]
- Jimenez M, Tsukamoto K, Hearing VJ. Tyrosinases from two different loci are expressed by normal and by transformed melanocytes. J Biol Chem. 1991;266:1147–1156. [PubMed] [Google Scholar]
- Jimenez-Cervantes C, Valverde P, Garcia-Borron JC, Solano F, Lozano JA. Improved tyrosinase activity stains in polyacrylamide electrophoresis gels. Pigment Cell Res. 1993;6:394–399. doi: 10.1111/j.1600-0749.1993.tb00621.x. [DOI] [PubMed] [Google Scholar]
- King RA, Lewis RA, Townsend D, Zelickson A, Olds DP, Brumbaugh J. Brown oculocutaneous albinism. Clinical, ophthalmological, and biochemical characterization. Ophthalmology. 1985;92:1496–1505. doi: 10.1016/s0161-6420(85)33832-0. [DOI] [PubMed] [Google Scholar]
- Kugelman TP, Van Scott EJ. Tyrosinase activity in melanocytes of human albinos. J Invest Dermatol. 1968;37:73–76. [PubMed] [Google Scholar]
- Lee ST, Nicholls RD, Bundey S, Laxova R, Musarella M, Spritz RA. Mutations of the P gene in oculocutaneous albinism, ocular albinism, and Prader-Willi syndrome plus albinism. N Engl J Med. 1994;330:529–534. doi: 10.1056/NEJM199402243300803. [DOI] [PubMed] [Google Scholar]
- Lee ST, Nicholls RD, Jong MT, Fukai K, Spritz RA. Organization and sequence of the human P gene and identification of a new family of transport proteins. Genomics. 1995;26:354–363. doi: 10.1016/0888-7543(95)80220-g. [DOI] [PubMed] [Google Scholar]
- Manga P, Boissy RE, Pifko-Hirst S, Zhou B-K, Orlow SJ. Mislocalization of melanosomal proteins in melanocytes from mice with oculocutaneous albinism type 2. Exp Eye Res. 2001;72:695–710. doi: 10.1006/exer.2001.1006. [DOI] [PubMed] [Google Scholar]
- Manga P, Orlow SJ. Induction of melanin synthesis by bafilomycin A1 is a function of pink-eyed dilution protein expression. Pigment Cell Res. 2001;14:362–367. doi: 10.1034/j.1600-0749.2001.140508.x. [DOI] [PubMed] [Google Scholar]
- Moyer FH. Genetic variations in the fine structure and ontogeny of mouse melanin granules. Am Zool. 1966;6:43–66. doi: 10.1093/icb/6.1.43. [DOI] [PubMed] [Google Scholar]
- Negroiu G, Branza-Nichita N, Petrescu AJ, Dwek RA, Petrescu SM. Protein specific N-glycosylation of tyrosinase and tyrosinase-related protein-1 in B16 mouse melanoma cells. Biochem J. 1999;344:659–665. [PMC free article] [PubMed] [Google Scholar]
- Orlow SJ, Brilliant MH. The pink-eyed dilution locus controls the biogenesis of melanosomes and levels of melanosomal proteins in the eye. Exp Eye Res. 1999;68:147–154. doi: 10.1006/exer.1998.0599. [DOI] [PubMed] [Google Scholar]
- Pomerantz SH. l-Tyrosine-3,5-3H assay for tyrosinase development in skin of newborn hamsters. Science. 1969;164:838–839. doi: 10.1126/science.164.3881.838. [DOI] [PubMed] [Google Scholar]
- Potterf SB, Furumura M, Sviderskaya EV, Santis C, Bennett DC, Hearing VJ. Normal tyrosine transport and abnormal tyrosinase routing in pink-eyed dilution melanocytes. Exp Cell Res. 1998;244:319–326. doi: 10.1006/excr.1998.4173. [DOI] [PubMed] [Google Scholar]
- Puri N, Gardner JM, Brilliant MH. Aberrant pH of melanosomes in pink-eyed dilution (p) mutant melanocytes. J Invest Dermatol. 2000;115:607–613. doi: 10.1046/j.1523-1747.2000.00108.x. [DOI] [PubMed] [Google Scholar]
- Ramsay M, Colman MA, Stevens G, Zwane E, Kromberg J, Farrall M, Jenkins T. The tyrosinase-positive oculocutaneous albinism locus maps to chromosome 15q11.2-q12. Am J Hum Genet. 1992;51:879–884. [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Tenza D, Murphy DM, Berson JF, Marks MS. Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J Cell Biol. 2001;152:809–824. doi: 10.1083/jcb.152.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinchik EM, Bultman SJ, Horsthemke B, Lee ST, Strunk KM, Spritz RA, Avidano KM, Jong MTC, Nicholls RD. A gene for the mouse pink-eyed dilution locus and for human type II oculocutaneous albinism. Nature. 1993;361:72–76. doi: 10.1038/361072a0. [DOI] [PubMed] [Google Scholar]
- Rittenhouse E. Genetic effects on fine structure and development of pigment granules in mouse hair bulb melanocytes II. The c and p loci, and ddpp interaction. Dev Biol. 1968;17:366–381. doi: 10.1016/0012-1606(68)90070-5. [DOI] [PubMed] [Google Scholar]
- Rosemblat S, Durham-Pierre D, Gardner JM, Nakatsu Y, Brilliant MH, Orlow SJ. Identification of a melanosomal membrane protein encoded by the pink eyed dilution (type II oculocutaneous albinism) gene. Proc Natl Acad Sci USA. 1994;91:12071–12075. doi: 10.1073/pnas.91.25.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosemblat S, Sviderskaya E, Easty DJ, Wilson A, Kwon BS, Bennett DC, Orlow SJ. Melanosomal defects in melanocytes from mice lacking expression of the pink-eyed dilution gene: correction by culture in the presence of excess tyrosine. Exp Cell Res. 1998;239:344–352. doi: 10.1006/excr.1997.3901. [DOI] [PubMed] [Google Scholar]
- Russell ES. A quantitative histological study of the pigment found in the coat-color mutants of the house-mouse. IV. The nature of the genic effects of five major allelic series. Genetics. 1949;34:146–166. doi: 10.1093/genetics/34.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiji M, Shimao K, Birbeck MSC, Fitzpatrick TB. Subcellular localization of melanin biosynthesis. Ann NY Acad Sci. 1963;100:497–533. [PubMed] [Google Scholar]
- Shen B, Rosenberg B, Orlow SJ. Intracellular distribution and late endosomal effects of the ocular albinism type 1 gene product: consequences of disease-causing mutations and implications for melanosome biogenesis. Traffic. 2001;2:202–211. doi: 10.1034/j.1600-0854.2001.020306.x. [DOI] [PubMed] [Google Scholar]
- Sprong H, Degroote S, Claessens T, van Drunen J, Oorschot V, Westerink BH, Hirabayashi Y, Klumperman J, van Der Sluijs P, van Meer G. Glycosphingolipids are required for sorting melanosomal proteins in the Golgi complex. J Cell Biol. 2001;155:369–380. doi: 10.1083/jcb.200106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sviderskaya EV, Bennett DC, Ho L, Bailin T, Lee ST, Spritz RA. Complementation of hypopigmentation in p-mutant (pink-eyed dilution) mouse melanocytes by normal human p cDNA, and defective complementation by OCA2 mutant sequences. J Invest Dermatol. 1997;108:30–34. doi: 10.1111/1523-1747.ep12285621. [DOI] [PubMed] [Google Scholar]
- Toyofuku K, Wada I, Hirosaki K, Park JS, Hori Y, Jimbow K. Promotion of tyrosinase folding in Cos 7 cells by calnexin. J Biochem. 1999;125:82–89. doi: 10.1093/oxfordjournals.jbchem.a022272. [DOI] [PubMed] [Google Scholar]
- Toyofuku K, Wada I, Spritz RA, Hearing VJ. The molecular basis of oculocutaneous albinism type 1 (OCA1): sorting failure and degradation of mutant tyrosinases results in a lack of pigmentation. Biochem J. 2001;355:259–269. doi: 10.1042/0264-6021:3550259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayasaradhi S, Xu Y, Bouchard B, Houghton AN. Intracellular sorting and targeting of melanosomal membrane proteins: identification of signals for sorting the human brown locus protein, gp75. J Cell Biol. 1995;130:807–820. doi: 10.1083/jcb.130.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B-K, Kobayashi T, Donatien PD, Bennett DC, Hearing VJ, Orlow SJ. Identification of a melanosomal matrix protein encoded by the murine si (silver) locus) using “organelle scanning”. Proc Natl Acad Sci USA. 1994;91:7076–7080. doi: 10.1073/pnas.91.15.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]