Abstract
1. In four awake dogs we measured EMG activity of three inspiratory and four expiratory muscles during sustained central chemoreceptor stimulation (CO2 inhalation), and peripheral chemoreceptor stimulation (intravenous infusion of almitrine bismesylate (almitrine)). By using this selective pharmacological stimulation of the peripheral chemoreceptors and reversibly cold-blocking pulmonary stretch receptors, we were able to determine the effects of each type of stimulation on respiratory muscle recruitment in the absence of such complicating influences as pulmonary stretch receptor feedback, cerebral hypoxia or hypocapnia, and differences in breathing pattern. 2. During 10 min of steady-state hyperpnoea (minute ventilation VI, approximately twice eupnoea) caused by either hypercapnia or isocapnic stimulation of the carotid bodies with almitrine, all three inspiratory and all four expiratory muscles demonstrated significant and sustained elevations in EMG activity. 3. With both types of chemoreceptor stimulation, as tidal volume, VT, increased, so did the mean electrical activities of the crural diaphragm (r = 0.88), costal diaphragm (r = 0.93), parasternals (r = 0.82), triangularis sterni (r = 0.74), transversus abdominis (r = 0.77), external obliques (r = 0.68) and internal intercostals (r = 0.75). 4. In each dog, the response of ventilation and of the diaphragmatic EMG to a given level of central or peripheral chemoreceptor stimulation is highly reproducible from one test day to the next. On the other hand, accessory inspiratory and expiratory abdominal and rib cage muscles in two of the four dogs showed highly significant changes from day to day in the amount of their EMG activity at any given VT. 5. During steady-state ventilatory stimulation, 2 min intervals were chosen during which the two types of chemoreceptor stimulation had caused hyperpnoeas with similar values for VT, total time per breath (TTOT) and inspiratory time divided by the total time (TI/TTOT). Comparison of EMG activities during these matched hyperpnoeas revealed that there were no differences in the activities of any of the muscles between the two forms of stimulation. We conclude that peripheral chemoreceptor stimulation causes significant and sustained recruitment of expiratory muscles even in the absence of pulmonary feedback and that both expiratory and inspiratory muscles are recruited to the same extent during peripheral chemoreceptor stimulation as they are during an identical hyperpnoea caused by central chemoreceptor stimulation.
Full text
PDF





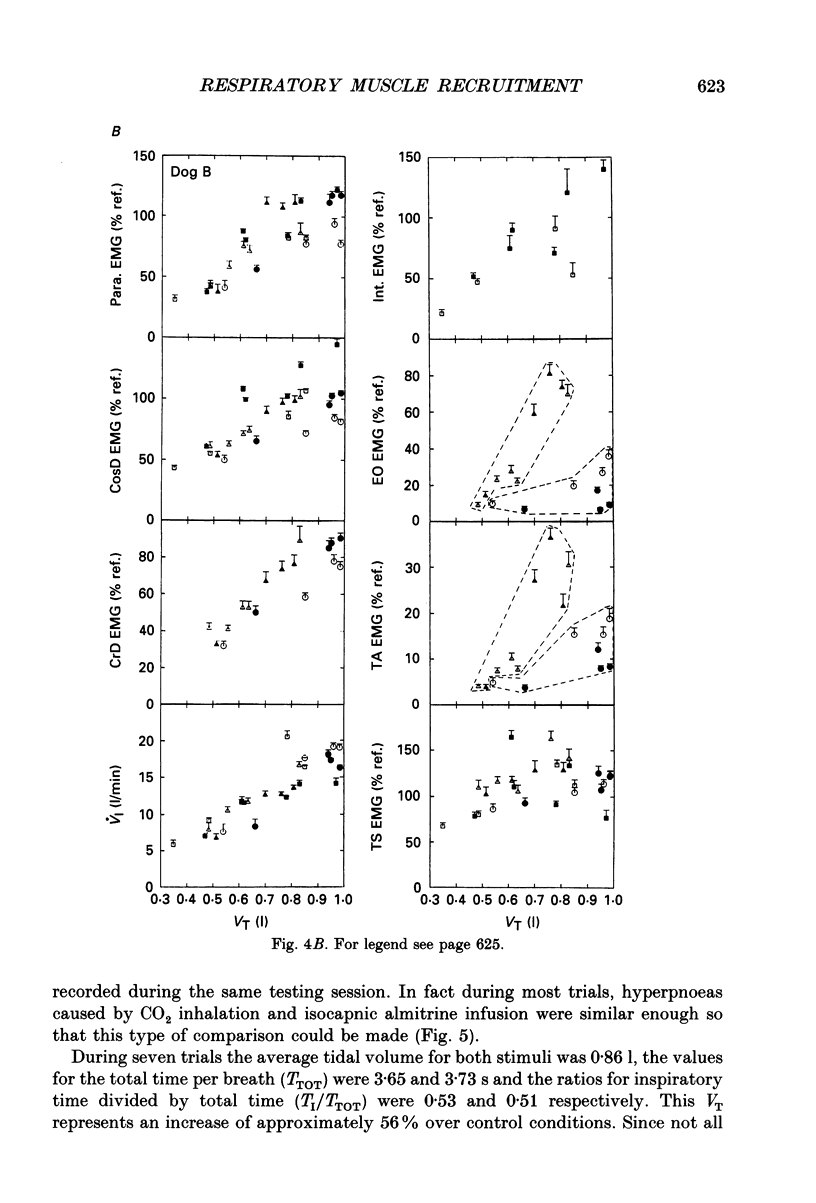
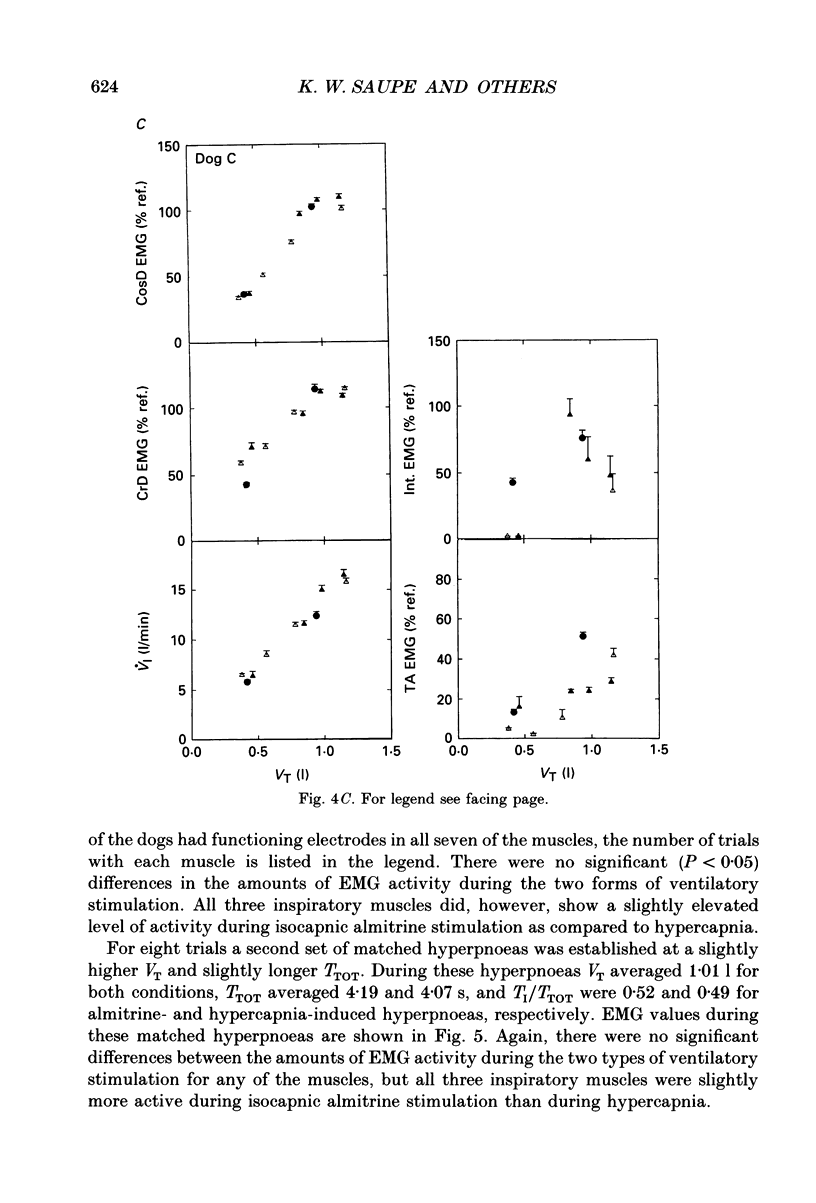
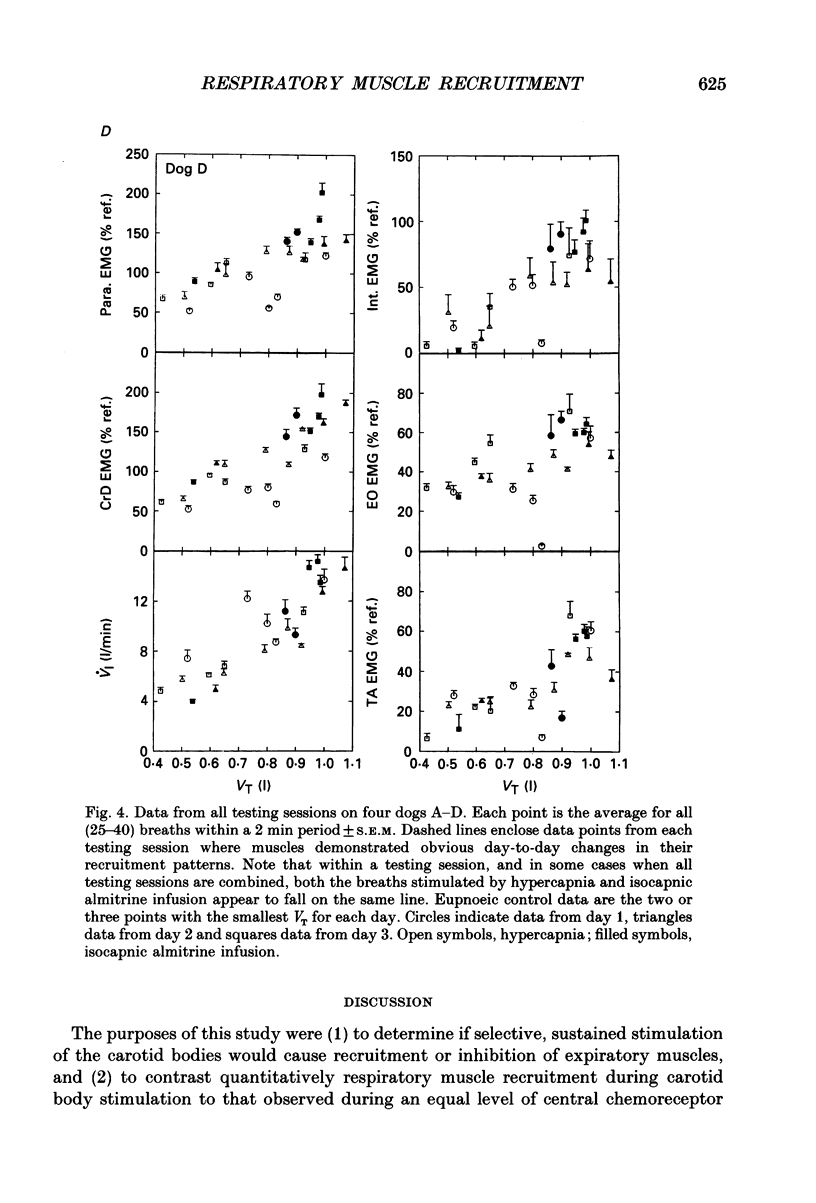





Selected References
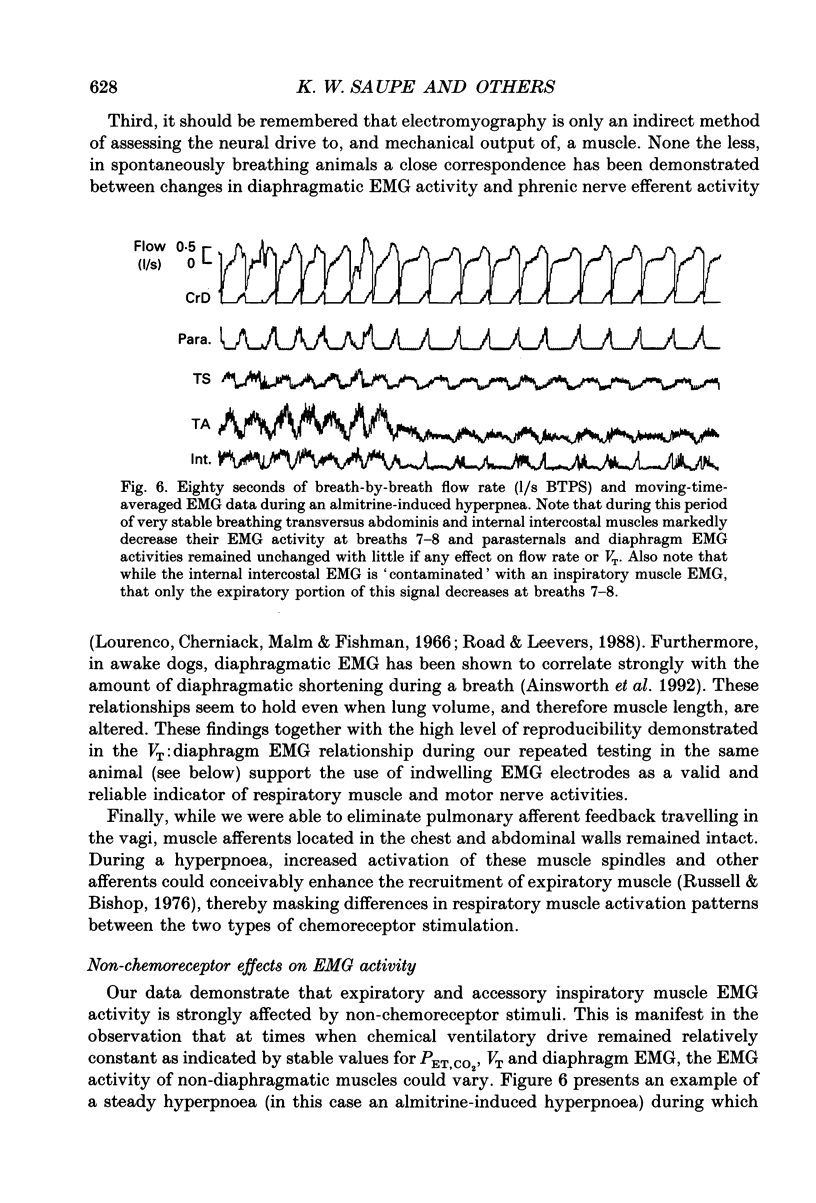
These references are in PubMed. This may not be the complete list of references from this article.
- Ainsworth D. M., Smith C. A., Eicker S. W., Henderson K. S., Dempsey J. A. The effects of chemical versus locomotory stimuli on respiratory muscle activity in the awake dog. Respir Physiol. 1989 Nov;78(2):163–176. doi: 10.1016/0034-5687(89)90049-2. [DOI] [PubMed] [Google Scholar]
- Bisgard G. E. The response of few-fiber carotid chemoreceptor preparations to almitrine in the dog. Can J Physiol Pharmacol. 1981 Apr;59(4):396–401. doi: 10.1139/y81-063. [DOI] [PubMed] [Google Scholar]
- Brice A. G., Forster H. V., Pan L. G., Lowry T. F., Murphy C. L. Respiratory muscle electromyogram responses to acute hypoxia in awake ponies. J Appl Physiol (1985) 1990 Mar;68(3):1024–1032. doi: 10.1152/jappl.1990.68.3.1024. [DOI] [PubMed] [Google Scholar]
- De Backer W., Vermeire P., Bogaert E., Janssens E., Van Maele R. Almitrine has no effect on gas exchange after bilateral carotid body resection in severe chronic airflow obstruction. Bull Eur Physiopathol Respir. 1985 Sep-Oct;21(5):427–432. [PubMed] [Google Scholar]
- De Troyer A., Ninane V. Effect of posture on expiratory muscle use during breathing in the dog. Respir Physiol. 1987 Mar;67(3):311–322. doi: 10.1016/0034-5687(87)90061-2. [DOI] [PubMed] [Google Scholar]
- Duron B., Marlot D. Intercostal and diaphragmatic electrical activity during wakefulness and sleep in normal unrestrained adult cats. Sleep. 1980;3(3-4):269–280. doi: 10.1093/sleep/3.3-4.269. [DOI] [PubMed] [Google Scholar]
- Eldridge F. L. Expiratory effects of brief carotid sinus nerve and carotid body stimulations. Respir Physiol. 1976 May;26(3):395–410. doi: 10.1016/0034-5687(76)90009-8. [DOI] [PubMed] [Google Scholar]
- Farkas G. A., Baer R. E., Estenne M., De Troyer A. Mechanical role of expiratory muscles during breathing in upright dogs. J Appl Physiol (1985) 1988 Mar;64(3):1060–1067. doi: 10.1152/jappl.1988.64.3.1060. [DOI] [PubMed] [Google Scholar]
- Fregosi R. F., Knuth S. L., Ward D. K., Bartlett D., Jr Hypoxia inhibits abdominal expiratory nerve activity. J Appl Physiol (1985) 1987 Jul;63(1):211–220. doi: 10.1152/jappl.1987.63.1.211. [DOI] [PubMed] [Google Scholar]
- Gilmartin J. J., Ninane V., De Troyer A. Abdominal muscle use during breathing in the anesthetized dog. Respir Physiol. 1987 Nov;70(2):159–171. doi: 10.1016/0034-5687(87)90047-8. [DOI] [PubMed] [Google Scholar]
- Ledlie J. F., Pack A. I., Fishman A. P. Effects of hypercapnia and hypoxia on abdominal expiratory nerve activity. J Appl Physiol Respir Environ Exerc Physiol. 1983 Nov;55(5):1614–1622. doi: 10.1152/jappl.1983.55.5.1614. [DOI] [PubMed] [Google Scholar]
- Leevers A. M., Road J. D. Mechanical response to hyperinflation of the two abdominal muscle layers. J Appl Physiol (1985) 1989 May;66(5):2189–2195. doi: 10.1152/jappl.1989.66.5.2189. [DOI] [PubMed] [Google Scholar]
- Lourenço R. V., Cherniack N. S., Malm J. R., Fishman A. P. Nervous output from the respiratory center during obstructed breathing. J Appl Physiol. 1966 Mar;21(2):527–533. doi: 10.1152/jappl.1966.21.2.527. [DOI] [PubMed] [Google Scholar]
- Marek W., Prabhakar N. R. Electrical stimulation of arterial and central chemosensory afferents at different times in the respiratory cycle of the cat: II. Responses of respiratory muscles and their motor nerves. Pflugers Arch. 1985 Apr;403(4):422–428. doi: 10.1007/BF00589256. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Tenney S. M. Hypoxia-induced tachypnea in carotid-deafferented cats. Respir Physiol. 1975 Jan;23(1):31–39. doi: 10.1016/0034-5687(75)90069-9. [DOI] [PubMed] [Google Scholar]
- Neubauer J. A., Melton J. E., Edelman N. H. Modulation of respiration during brain hypoxia. J Appl Physiol (1985) 1990 Feb;68(2):441–451. doi: 10.1152/jappl.1990.68.2.441. [DOI] [PubMed] [Google Scholar]
- Phillipson E. A., Hickey R. F., Bainton C. R., Nadel J. A. Effect of vagal blockade on regulation of breathing in conscious dogs. J Appl Physiol. 1970 Oct;29(4):475–479. doi: 10.1152/jappl.1970.29.4.475. [DOI] [PubMed] [Google Scholar]
- Road J. D., Leevers A. M. Effect of lung inflation on diaphragmatic shortening. J Appl Physiol (1985) 1988 Dec;65(6):2383–2389. doi: 10.1152/jappl.1988.65.6.2383. [DOI] [PubMed] [Google Scholar]
- Russell J. A., Bishop B. Vagal afferents essential for abdominal muscle activity during lung inflation in cats. J Appl Physiol. 1976 Sep;41(3):310–315. doi: 10.1152/jappl.1976.41.3.310. [DOI] [PubMed] [Google Scholar]
- Sears T. A., Berger A. J., Phillipson E. A. Reciprocal tonic activation of inspiratory and expiratory motoneurones by chemical drives. Nature. 1982 Oct 21;299(5885):728–730. doi: 10.1038/299728a0. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Ainsworth D. M., Henderson K. S., Dempsey J. A. Differential responses of expiratory muscles to chemical stimuli in awake dogs. J Appl Physiol (1985) 1989 Jan;66(1):384–391. doi: 10.1152/jappl.1989.66.1.384. [DOI] [PubMed] [Google Scholar]
- St John W. M. Respiratory neuron responses to hypercapnia and carotid chemoreceptor stimulation. J Appl Physiol Respir Environ Exerc Physiol. 1981 Oct;51(4):816–822. doi: 10.1152/jappl.1981.51.4.816. [DOI] [PubMed] [Google Scholar]


