Abstract
Oxidation of low-density lipoprotein (LDL) may play an important role in atherosclerosis. We studied the effects of bicarbonate/CO2 and phosphate buffer systems on metal ion-catalyzed oxidation of LDL to malondialdehyde (MDA) and to protein carbonyl and MetO derivatives. Our results revealed that LDL oxidation in mixtures containing free iron or heme derivatives was much greater in bicarbonate/CO2 compared with phosphate buffer. However, when copper was substituted for iron in these mixtures, the rate of LDL oxidation in both buffers was similar. Iron-catalyzed oxidation of LDL was highly sensitive to inhibition by phosphate. Presence of 0.3-0.5 mM phosphate, characteristic of human serum, led to 30-40% inhibition of LDL oxidation in bicarbonate/CO2 buffer. Iron-catalyzed oxidation of LDL to MDA in phosphate buffer was inhibited by increasing concentrations of albumin (10-200 μM), whereas MDA formation in bicarbonate/CO2 buffer was stimulated by 10-50 μM albumin but inhibited by higher concentrations. However, albumin stimulated the oxidation of LDL proteins to carbonyl derivatives at all concentrations examined in both buffers. Conversion of LDL to MDA in bicarbonate/CO2 buffer was greatly stimulated by ADP, ATP, and EDTA but only when EDTA was added at a concentration equal to that of iron. At higher than stoichiometric concentrations, EDTA prevented oxidation of LDL. Results of these studies suggest that interactions between bicarbonate and iron or heme derivatives leads to complexes with redox potentials that favor the generation of reactive oxygen species and/or to the generation of highly reactive CO2 anion or bicarbonate radical that facilitates LDL oxidation.
It has been suggested that the oxidation of low-density lipoprotein (LDL) in the vessel wall may be an early event in atherosclerosis (1-5). Some epidemiological and histochemical studies suggest that metal ion-catalyzed reactions might be involved in LDL oxidation, but these data are controversial (1, 5-8).
The intracellular and serum concentrations of bicarbonate anion 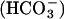 in humans are in the range of 14.7-25 mM and are in equilibrium with 5% CO2 (1.3 mM) to yield pH values of 7.2 and 7.4, respectively. As such, they constitute the major physiological buffers. A number of in vitro studies have been carried out to examine the effects of transition metal ions and physiological iron-containing substances (hemoglobin and others) on the oxidation of LDL. However, these studies have been carried out in nonphysiological buffers, such as phosphate. Previous studies (9-12) have shown that oxidation of free amino acids by metal ion-catalyzed oxidation systems is greatly stimulated by bicarbonate/CO2 buffers. In this work, we compared the effects of bicarbonate/CO2 buffers with phosphate buffer systems on the metal ion-catalyzed oxidation of LDL to malondialdehyde (MDA) and protein carbonyl and methionine sulfoxide (MetO) derivatives.
in humans are in the range of 14.7-25 mM and are in equilibrium with 5% CO2 (1.3 mM) to yield pH values of 7.2 and 7.4, respectively. As such, they constitute the major physiological buffers. A number of in vitro studies have been carried out to examine the effects of transition metal ions and physiological iron-containing substances (hemoglobin and others) on the oxidation of LDL. However, these studies have been carried out in nonphysiological buffers, such as phosphate. Previous studies (9-12) have shown that oxidation of free amino acids by metal ion-catalyzed oxidation systems is greatly stimulated by bicarbonate/CO2 buffers. In this work, we compared the effects of bicarbonate/CO2 buffers with phosphate buffer systems on the metal ion-catalyzed oxidation of LDL to malondialdehyde (MDA) and protein carbonyl and methionine sulfoxide (MetO) derivatives.
Materials and Methods
Materials. Hemin, human methemoglobin, human recombinant albumin, and bovine heart cytochrome c were obtained from Sigma. The 2,4-dinitrophenylhydrazine was purchased from Mallinckrodt. The 2,2′-azobis(2-amidinopropane) dihydrochloride was supplied from Wako Pure Chemical (Osaka). All other chemicals were of reagent grade. LDL (density 1.019-1.063 g/ml) was separated from fresh human plasma, obtained from a healthy volunteer with informed consent after fasting for 8 h, by the ultracentrifugation method (13).
Metal Ion-Catalyzed Oxidation of LDL and Analysis of Lipid Peroxidation. Bicarbonate/CO2 buffer containing 23.3 mM bicarbonate and 100 mM NaCl (pH 7.6) was prepared by treating the buffer with chelating resin (Chelex 100, Sigma) and equilibrated by bubbling with 5% CO2/95% air at 37°C just before use. For the metal ion-catalyzed experiments, LDL (200 μg of protein) was dispersed in 1 ml of bicarbonate/CO2 buffer and preincubated in 5% CO2/95% air at 37°C for 30 min. The LDL oxidation was initiated by adding either FeCl2 (100 μM), hemin (2 μM), hemoglobin (1 μM), cytochrome c (10 μM), or CuCl2 (1 μM) in the presence or absence of ascorbate (50 μM), H2O2 (200 μM), ADP (100 or 400 μM), EDTA (100 or 400 μM), deferoxamine (100 or 400 μM), or albumin (10-200 μM). The comparative experiments were carried out in phosphate buffer (23.3 mM, pH 7.6) containing 100 mM NaCl and equilibrated with 100% air at 37°C. At the indicated times, the pH of the reaction mixture was checked, and the concentration of MDA generated by the decomposition of lipid hydroperoxides in the reaction mixture was determined by HPLC monitoring changes in fluorescent intensity at 553 nm with excitation wave length set at 515 nm (14).
Protein Carbonyl Analysis. Oxidized LDL was derivatized with 2,4-dinitrophenylhydrazine and subjected to SDS/PAGE in a 4-15% gradient acrylamide slab gel. The 2,4-dinitrophenylhydrazine-derivatized proteins were detected by immunoblotting with rabbit anti-dinitrophenyl antisera (DakoCytomation, Glostrup, Denmark) as described in ref. 15. Immunoreactive species were visualized by using the Odyssey infrared imaging system (Li-Cor, Lincoln, NE) with IRDye 800-conjugated donkey anti-rabbit IgG (Rockland, Gilbertsville, PA) as a secondary Ab.
Determination of MetO. Oxidized LDL was treated with CNBr, which cleaves peptide bonds on the carboxyl side of Met to yield homoserine (16). When Met is oxidized to MetO, CNBr fails to cleave such bonds (17). Samples with and without CNBr treatment were hydrolyzed by hydrochloric acid, and the resulting amino acid composition was analyzed by using HPLC techniques (18). The amount of MetO was calculated from the difference in peak areas obtained by integrating the corresponding peaks.
Peroxyl Radical-Mediated Oxidation of LDL. LDL (100 μg of protein) in 23.3 mM bicarbonate/CO2 or phosphate buffer (pH 7.6) described above was oxidized by 500 μM 2,2′-azobis(2-amidinopropane) dihydrochloride, a hydrophilic peroxyl radical generator, at 37°C in the presence of 100 μM diethylenetriaminepentaacetic acid, which prevents the decomposition of lipid hydroperoxides from contaminating metal ion. At the indicated times, cholesteryl ester hydroperoxides in the reaction mixture were determined by using HPLC and changes in absorbance at 235 nm as described in ref. 19.
Results
Lipid Peroxidation of LDL Mediated by Iron in Bicarbonate and Phosphate Buffers. The effects of ascorbate and H2O2 on iron-mediated oxidation of LDL to MDA, in both bicarbonate/CO2 and inorganic phosphate buffers, is illustrated by data summarized in Fig. 1. Fig. 1A shows that very little MDA (<0.3 μM) is formed during a 16-h incubation with FeCl2 alone in either buffer. However, further addition of either ascorbate (Fig. 1B) or H2O2 (Fig. 1C) or both ascorbate and H2O2 (Fig. 1D) leads to substantial increases in the rate of MDA formation in bicarbonate/CO2 but not in phosphate buffer. Moreover, very little MDA (<0.05 μM) is formed in either buffer when LDL is incubated with H2O2 in the absence of iron (data not shown).
Fig. 1.
Comparison of bicarbonate and phosphate buffers on lipid peroxidation of LDL by iron-mediated oxidation system. LDL (200 μg of protein) was oxidized by 100 μM FeCl2 (A), 100 μM FeCl2 with 50 μM ascorbate (B), 100 μM FeCl2 with 200 μMH2O2 (C), or 100 μM FeCl2 with 50 μM ascorbate and 200 μM H2O2 (D) in 1 ml of 23.3 mM bicarbonate/CO2 buffer (▴) equilibrated with 5% CO2/95% air or 23.3 mM phosphate buffer (○) at 37°C, pH 7.6, as described in Materials and Methods. At times indicated on the abscissa, aliquots were tested for the content of MDA by changes in fluorescent intensity of samples separated with HPLC.
Protein Oxidation of LDL Mediated by Iron in Bicarbonate and Phosphate Buffers. Fig. 2A shows the protein carbonyl patterns obtained after oxidation of LDL by H2O2 in the presence or absence of iron and ascorbate. After a 16-h incubation in the mixtures shown at the bottom of Fig. 2A, proteins were separated by slab gel electrophoresis, and protein bands containing carbonyl groups were detected by the immunoblotting technique. It is evident from these data that little protein oxidation occurs in phosphate buffer (lanes 1, 3, and 5) and that the generation of protein carbonyls in bicarbonate/CO2 buffer depends on the presence of both iron and H2O2 (lanes 2, 4, and 6). After gel electrophoresis of samples incubated in the presence of iron, ascorbate, and H2O2, the intensity of protein carbonyls in these bands was integrated by using the Odyssey infrared imaging system. Data in Fig. 2B show that there is a time-dependent increase in the protein carbonyl content of reactions carried out in bicarbonate/CO2 buffer but not in phosphate buffer. Data in Fig. 2C show that there is a substantial increase (≈4-fold) in the levels of oxidized methionine residues (MetO) after a 4-h incubation in either buffer. However, with further incubation, e.g., 8 and 12 h, the level of MetO continues to increase in bicarbonate/CO2 buffer but not in phosphate buffer.
Fig. 2.
Effect of bicarbonate and phosphate buffers on protein oxidation of LDL by iron-mediated oxidation system. Protein carbonyl (A and B) and MetO (C) formation were analyzed for protein oxidation in LDL. (A) After incubation of LDL for 16 h, under conditions noted at the bottom of the image and as described in Fig. 1, 170 ng of protein in each sample was subjected to slab gel electrophoresis, and bands containing carbonyl derivatives were visualized by reaction with anti-dinitrophenyl Abs. The reaction was carried out in either phosphate buffer (lanes 1, 3, and 5) or bicarbonate/CO2 buffer (lanes 2, 4, and 6); 100 μM FeCl2, 50 μM ascorbate, and/or 200 μM H2O2 were present when indicated. (B) Time-dependent accumulation of protein carbonyl during LDL oxidized by Fe(II)/ascorbate/H2O2 system in bicarbonate/CO2 buffer (▴) and in phosphate buffer (○). The relative protein carbonyl content was determined by the intensity of all immunoreactive protein carbonyl on the blotting membrane, monitored with the Odyssey infrared imaging system. (C) Time-dependent accumulation of MetO in LDL oxidized by Fe(II)/ascorbate/H2O2 system in phosphate buffer (light gray) and in bicarbonate/CO2 buffer (dark gray). Data are mean ± SD (n = 3). Statistical significance was assessed by using Student's t test; *, P < 0.05; **, P < 0.001.
Effect of pH and Buffer Concentration on LDL Oxidation. Results summarized in Fig. 3 show that at pH 7.4 there is very little effect of bicarbonate concentration (14.7 vs. 25 mM) on the rate of MDA formation during iron-mediated oxidation of LDL. However, the rate of LDL oxidation in 9.3 mM bicarbonate/CO2 buffer at pH 7.2 was considerably greater than at pH 7.4. Significantly, the rates of LDL oxidation in the phosphate buffer under all of these conditions were much lower than those observed in bicarbonate/CO2 buffer.
Fig. 3.
Effect of buffer concentration on iron-mediated oxidation of LDL. To obtain a pH of 7.4 for bicarbonate/CO2 buffer, 14.7 mM (A) and 25 mM (B) bicarbonate solution containing 100 mM NaCl were equilibrated with 5% CO2/95% and 8.5% CO2/91.5% air, respectively. To obtain a pH of 7.2 bicarbonate/CO2 buffer (C), 9.3 mM bicarbonate solution containing 100 mM NaCl was equilibrated with 5% CO2/95%. LDL was oxidized by Fe(II)/ascorbate/H2O2 system in the bicarbonate/CO2 buffers (▴). The reaction was carried out in either phosphate buffer (○) at pH 7.4 (A and B) and pH 7.2 (C). At the times indicated, aliquots of the reaction mixtures were assayed for MDA.
Inorganic Phosphate Inhibits LDL Oxidation in Bicarbonate Buffer. Iron-mediated oxidation of LDL to MDA is susceptible to inhibition by inorganic phosphate in a concentration-dependent manner at pH 7.4 (Fig. 4A). Interestingly, supplementation of the bicarbonate/CO2 buffer (14.7 mM) with an equivalent amount of phosphate leads to almost complete inhibition of LDL oxidation. Moreover, addition of phosphate at the normal serum concentration of phosphate (0.3-0.5 mM) leads to 30-40% inhibition of the activity observed in bicarbonate/CO2 buffer alone (Fig. 4B).
Fig. 4.
Phosphate inhibits iron-mediated oxidation of LDL in bicarbonate/CO2 buffer. (A) LDL was oxidized by Fe(II)/ascorbate/H2O2 system in 14.7 mM bicarbonate buffer equilibrated with 5% CO2/95% air in the presence of phosphate at 37°C, pH 7.4. At indicated times, MDA was measured as described in Materials and Methods. Phosphate concentration in various samples was varied as follows; no phosphate (○), 0.03 mM (▵), 0.11 mM (▿), 0.46 mM (▪), 1.84 mM (▴), 7.35 mM (♦), and 14.7 mM (•). (B) Replot of the data in A obtained after 4-h (□) and 16-h (▿) incubation as a function of phosphate concentration.
Effect of Albumin on Iron-Mediated Oxidation of LDL in Bicarbonate and Phosphate Buffers. Fig. 5 A-D shows that at low concentrations (10-50 μM), albumin stimulated the MDA formation by iron-mediated oxidation reactions carried out in bicarbonate/CO2 buffer (dark gray bars). However, at higher concentrations (100-200 μM), albumin inhibited significantly the MDA formation in bicarbonate/CO2 buffer. At all concentrations examined (10-200 μM), albumin inhibited the formation of MDA in phosphate buffer in a dose-dependent manner (light gray bars). It is noteworthy that addition of 200 μM albumin had no effect on the pH of either buffer. To examine the possibility that inhibition of MDA formation by high concentrations of albumin is due to preferential reaction of the reactive oxygen species with albumin protein, we examined the effects of albumin concentration on the generation of protein carbonyl derivatives by the Fe(II)/ascorbate/H2O2 system (Fig. 5E) in mixtures containing albumin alone (Right) and in mixtures containing both LDL and albumin (Center). It is evident that the oxidation of albumin in both mixtures increases with increasing albumin concentration in both bicarbonate/CO2 (lanes 2, 4, 6, and 8) and phosphate buffers (lanes 1, 3, 5, and 7). It is also evident from a comparison of results shown in Fig. 5E Right and Center that LDL stimulates the oxidation of albumin as indicated by the enhanced amount of protein carbonyl derivatives of protein fragments derived from albumin (Mr < 200 kDa), whereas albumin had little effect on the oxidation of LDL proteins (proteins of >200 kDa).
Fig. 5.
Effect of albumin on iron-mediated oxidation of LDL in bicarbonate and phosphate buffers. MDA and protein carbonyl levels were measured after exposure of LDL (200 μg of protein/ml) to various oxidation mixtures for 16 h in phosphate (light gray) or bicarbonate/CO2 buffer (dark gray) at 37°C, pH 7.6, in the presence or absence of albumin, as indicated on abscissa. The oxidation systems were as follows: 100 μM FeCl2 (A); 100 μM FeCl2 + 50 μM ascorbate (B); 100 μM FeCl2 + 200 μM H2O2 (C); and 100 μM FeCl2 + 50 μM ascorbate + 200 μM H2O2 (D). After oxidation, 2 μl of 18% SDS in 2 mM diethylenetriaminepentaacetic acid was added to 100 μl of the reaction mixture to stop the oxidation. Then, 10 μl of 10% trichloroacetic acid was mixed with the solution to precipitated proteins. After centrifugation, 56 μl of the supernatant was taken for analysis of MDA. (E) Protein carbonyls in LDL alone (Left), LDL with albumin (Center), and albumin alone (Right) oxidized by Fe(II)/ascorbate/H2O2 system for 16 h at pH 7.6 was analyzed by immunoblotting before trichloroacetic acid precipitation. Lane a was obtained with phosphate buffer, and lane b was obtained with bicarbonate buffer. Lanes 1, 3, 5, and 7 were obtained with 10, 50, 100, and 200 μM albumin, respectively, in phosphate buffer. Lanes 2, 4, 6, and 8 were obtained with 10, 50, 100, and 200 μM albumin, respectively, in bicarbonate/CO2 buffer.
Effect of Iron Chelators on LDL Oxidation. Oxidation of LDL to MDA by the Fe(II)/ascorbate/H2O2 system is stimulated severalfold by the presence of ADP when added at a concentration equivalent to the concentration of FeCl2 (100 μM) and even much more so by the addition of 400 μM ADP when reactions are carried out in bicarbonate/CO2 buffer (pH 7.6) but not in phosphate buffer (Fig. 6A). In contrast, addition of 100 μM of EDTA to the oxidation system leads to a great enhancement of MDA formation in both bicarbonate/CO2 and phosphate buffers, but at 400 μM there is little or no effect of EDTA on MDA generation in either buffer (Fig. 6B). Moreover, when reactions were carried out in the absence of ascorbate, addition of 400 μM ADP had no effect in either buffer system, and stimulation of MDA formation by 100 μM EDTA was considerably lower in both buffers (data not shown). Results of other studies showed that, when added at either 100 or 400 μM concentrations, deferoxamine inhibited the oxidation of LDL to MDA (data not shown). Addition of EDTA at a concentration equal to the iron concentration led to a great increase in protein carbonyl content of LDL in both buffer systems (Fig. 6C, lanes 5 and 6). However addition of ADP at four times the iron concentration led to much greater stimulation of protein oxidation in bicarbonate buffer (lane 4) than in phosphate buffer (lane 2), compared with the controls (lanes 1 and 2). We also tested the effect of ATP (400 μM) on the oxidation of LDL by Fe(II)/ascorbate/H2O2 system. ATP strongly stimulated MDA formation and the MDA generated was more than twice to that of observed with ADP after a 4-h incubation (data not shown).
Fig. 6.
Effect of iron chelators and buffer composition on iron-mediated oxidation of LDL. MDA formation in LDL oxidized by Fe(II)/ascorbate/H2O2 system for 16 h at pH 7.6, 37°C, in the presence of either ADP (A) or EDTA (B). The data in A and B were obtained in the presence of 400 μM ADP or EDTA in bicarbonate/CO2 buffer (▴) or phosphate buffer (○) or in the presence of 100 μM ADP or EDTA in bicarbonate/CO2 buffer (▪)or phosphate buffer (▿). (C) Protein carbonyl formation in LDL oxidized by Fe(II)/ascorbate/H2O2 system for 16 h at pH 7.6 was analyzed by Western blot as described in Fig. 1. Protein (170 ng) was used for all lanes in Left and 57 ng of protein was used for all lanes in Right. The oxidation was carried out in the presence or absence of 400 μM ADP or 100 μM EDTA as indicated in either phosphate buffer (lanes 1, 3, 5, and 7) or bicarbonate/CO2 buffer (lanes 2, 4, 6, 8).
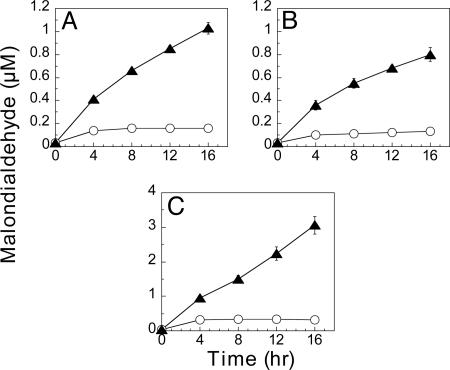
Oxidation of LDL by Hemin and Heme-Containing Proteins. Oxidation of LDL to MDA and protein carbonyl derivatives occurs also when FeCl2 in the ascorbate/H2O2 mixture is replaced by hemin, hemoglobin, or cytochrome c. Oxidation in the presence of any one of these heme compounds is greater in bicarbonate/CO2 buffer than in phosphate buffer (Fig. 7). In the absence of ascorbate LDL oxidations by hemin or hemoglobin were slightly decreased, but oxidation by cytochrome c in bicarbonate/CO2 buffer was about the same as that observed in phosphate buffer (data not shown).
Fig. 7.
Oxidation of LDL by hemin and heme iron proteins in bicarbonate and phosphate buffers. MDA was determined after oxidation of LDL (200 μg protein) in mixtures containing 50 μM ascorbate, 200 μM H2O2, and either 2 μM hemin (A), 1.0 μM hemoglobin (B), or 10 μM cytochrome c (C) in phosphate buffer (○), or bicarbonate/CO2 buffer (▴), at pH 7.6. (D-F) After oxidation, the protein carbonyl content of these mixtures was determined by Western blot, as described in Fig. 1, after oxidation mediated by hemin (D), hemoglobin (E), and cytochrome c (F). Lanes 1 and 2 show results obtained in phosphate and bicarbonate/CO2 buffers, respectively.
Oxidation of LDL by Peroxyl Radicals. Oxidation of LDL by peroxyl radicals generated during incubation with 2,2′-azobis(2-amidinopropane) dihydrochloride in aqueous solution leads to formation of cholesteryl ester hydroperoxide in both bicarbonate/CO2 and phosphate buffers (Fig. 8).
Fig. 8.
Peroxyl radical-mediated oxidation of LDL in bicarbonate and phosphate buffers. LDL was oxidized by 500 μM 2,2′-azobis(2-amidinopropane) dihydrochloride in phosphate buffer (○) or bicarbonate/CO2 buffer (▴), with pH 7.6 at 37°C, in the presence of 100 μM diethylenetriaminepentaacetic acid. Cholesteryl ester hydroperoxide was determined by HPLC with UV (235 nm) detection.
Oxidation of LDL by Copper. In contrast to results obtained in the oxidation of LDL by various iron-dependent systems, the formation of MDA by copper(II) was the same in both bicarbonate/CO2 and phosphate buffers. The rate of Cu(II)-dependent oxidation was greatly increased by the presence of only H2O2 (Fig. 9B). However, when Cu(II) (Fig. 9A) or a mixture of Cu(II), ascorbate, and H2O2 (Fig. 9C) was used to oxidize LDL, an ≈4-h lag phase was observed before the large increase in MDA formation.
Fig. 9.
Effect of buffer composition on copper-mediated oxidation of LDL. MDA concentration was measured after oxidation of 200 μg of LDL protein in 1-ml mixtures at 37°C for the indicated time in phosphate (○) or bicarbonate/CO2 (▴) buffers at pH 7.6. Mixtures contained: 1.0 μM CuCl2 (A), 1.0 μM CuCl2 and 200 μMH2O2 (B), and 1.0 μM CuCl2, 50 μM ascorbate, and 200 μM H2O2 (C).
Discussion
Bicarbonate and/or CO2 has been shown to be implicated in several physiological processes as follows: (i) free radical generation from hydrogen peroxide catalyzed by copper/zinc superoxide dismutase (20-23); (ii) peroxynitrite-dependent nitration of protein Tyr residues (24); (iii) the ability of peroxynitrite to oxidize protein methionine residues to MetO (25); and (iv) metal-catalyzed oxidation of free amino acids to form α-ketoacids and ammonia (11). The effects of bicarbonate on the nitration and oxidation of LDL by peroxynitrite are controversial. Thomas et al. (26) reported that bicarbonate inhibits the oxidation of Lys and Trp residues in LDL by peroxynitrite, whereas Ferroni et al. (27) reported that bicarbonate stimulates Trp oxidation and formation of 3-nitrotyrosine, but inhibits lipid oxidation by peroxynitrite. Although metal ions are believed to contribute to oxidative stress in vivo (28), little or no information is available on a role of metal ions in LDL oxidation in bicarbonate/CO2 buffers. Therefore, we examined the effects of Fe(II), Cu(II), and some heme-iron-containing compounds on the oxidation of LDL in both bicarbonate/CO2 and phosphate buffers at various pHs. It is well known that bicarbonate buffers are the major buffers in animal cells and serum; however, almost all in vitro studies have been carried out in phosphate or another nonphysiological buffer system, mainly because the development of bicarbonate buffers in the physiological range of pH is more difficult to control. Bicarbonate, in equilibrium with 5% CO2, constitutes the major physiological buffer system. At intracellular bicarbonate concentrations of 14.7 mM, the pH is 7.2 and, at serum bicarbonate concentrations of 25 mM, the pH is 7.4. In our in vitro studies, equilibration of sodium bicarbonate solutions at these concentrations with 5% CO2 yielded pH values of 7.4 and 7.6, respectively. Therefore, to assess the effects of bicarbonate buffers on metal-catalyzed oxidation of LDL, we carried out studies at several different concentrations of bicarbonate after equilibration with 5% CO2/95% air and also in 25 mM bicarbonate equilibrated with 8.5% CO2 to obtain a pH of 7.4. For comparison, we also examined the effects of phosphate buffer at concentrations and pH values comparable to those of the bicarbonate/CO2 buffers. Results of in vitro studies summarized here show that iron-mediated oxidation of LDL to MDA, protein carbonyls, and MetO derivatives is much greater when reactions are carried out in bicarbonate/CO2 buffer, as compared with phosphate buffer, in the physiological pH range, at 37°C (Figs. 1, 2, 3). Because human serum and most tissues contain low levels of phosphate, we studied the effect of phosphate concentration on LDL oxidation in 14.7 mM bicarbonate/CO2 buffer (pH 7.4). We found that lipid peroxidation was strongly inhibited by increasing concentrations of phosphate (0.03-14.7 mM). Curiously, at physiological concentrations (0.3-0.5 mM), inorganic phosphate led to 30-40% inhibition of MDA formation and, at a level stoichiometric with the bicarbonate (14.7 mM), the stimulatory effect of bicarbonate was almost completely eliminated (Fig. 4A).
Metal-ion chelators have been shown to affect the ability of iron to catalyze the oxidation of lipids (29-31). In earlier studies (9), we demonstrated that any one of several different chelators stimulated iron-catalyzed oxidation of Leu in bicarbonate/CO2 buffer but only when added at concentrations less than or equal to the iron concentration. At higher than stoichiometric concentrations, all iron chelators (except ADP) led to complete inhibition of Leu oxidation. ADP was found to stimulate iron-catalyzed oxidation of Leu even at very high (1 mM) concentrations, possibly because of its relatively low affinity to form complex with iron. In the present study, we observed similar effects of ADP, ATP, and EDTA on the iron-catalyzed oxidation of LDL to form MDA and protein carbonyl derivatives (Fig. 6).
Others have shown that oxidation of LDL to MDA in phosphate buffer is enhanced by copper/albumin complexes when added at a 2:1 ratio (32); whereas higher concentrations of albumin were inhibitory (33, 34). We found that the iron-mediated oxidation of LDL to MDA in phosphate buffer was inhibited by albumin in a concentration-dependent manner. However, in bicarbonate/CO2 buffer, low concentrations of albumin (up to one-half the iron concentration) stimulated the iron-mediated generation of MDA, but at higher concentrations (up to twice the level of iron) albumin inhibited MDA formation (Fig. 5A-D). However, albumin exhibits no effect on iron-dependent oxidation to carbonyl derivatives in both bicarbonate/CO2 and phosphate buffers (Fig. 5E). To the contrary, as shown in Fig. 5, LDL enhances the formation of albumin carbonyl derivatives. This result could reflect secondary reactions of albumin with LDL-derived lipid peroxides or MDA (35). The observed increase in carbonyls in reactions containing albumin in the absence of LDL suggests that inhibition of MDA formation by albumin in phosphate buffer and by high concentrations of albumin in bicarbonate/CO2 buffer reflects preferential oxidation of albumin by reactive oxygen species generated by the iron-mediated oxidation system.
Stress-induced hemolysis of circulating erythrocytes leads to the release of hemoglobin (36). Both hemoglobin and its iron-containing degradation product, hemin, have been shown to facilitate LDL oxidation in the presence of H2O2 in vitro (37-41). Upon incubation with high concentrations of H2O2 in vitro, hemoglobin is degraded to form heme and free iron (28). In view of these findings, we carried out studies to compare the abilities of hemin, hemoglobin, and cytochrome c to oxidize LDL in bicarbonate/CO2 and phosphate buffers. We found that all three heme compounds catalyze the conversion of LDL to MDA and to protein carbonyl derivatives and that these oxidations were much greater in bicarbonate/CO2 buffer than in phosphate buffer (Fig. 7). It remains to be determined whether the observed effects are related to the release of free iron or heme compounds.
The demonstration that there is almost no difference between bicarbonate/CO2 and phosphate buffers on the oxidation of LDL by peroxyl radicals (Fig. 8) discounts the possibility that the stimulation of LDL oxidation by iron is due to differences in rates of oxidation by alkyl peroxide, but does not preclude the possibility that bicarbonate stimulates the ability of Fe(II) to generate formation of alkyl peroxides during LDL oxidation.
Finally, it is evident that the oxidation of LDL by Cu(II) is not significantly affected by the buffer composition but is dependent on H2O2 and is retarded by ascorbate (Fig. 9).
Results of these studies suggest that interactions between bicarbonate and iron or heme derivatives lead to complexes that have redox potentials that favor the generation of reactive oxygen species and/or to the generation of highly reactive carbonate radical anion, bicarbonate radical, or peroxymonocarbonate (42) that facilitate LDL oxidation.
Abbreviations: LDL, low-density lipoprotein; MDA, malondialdehyde; MetO, methionine sulfoxide.
References
- 1.Heinecke, J. W. (1998) Atherosclerosis 141, 1-15. [DOI] [PubMed] [Google Scholar]
- 2.Thomas, M. J. (2000) Curr. Opin. Lipidol. 11, 297-301. [DOI] [PubMed] [Google Scholar]
- 3.Chisolm, G. M. & Steinberg, D. (2000) Free Radical Biol. Med. 28, 1815-1826. [DOI] [PubMed] [Google Scholar]
- 4.Witztum, J. L. & Steinberg, D. (2001) Trends Cardiovasc. Med. 11, 93-102. [DOI] [PubMed] [Google Scholar]
- 5.Stocker, R. & Keaney, J. F., Jr. (2004) Physiol. Rev. 84, 1381-1478. [DOI] [PubMed] [Google Scholar]
- 6.Yuan, X. M. & Brunk, U. T. (1998) Apmis 106, 825-842. [DOI] [PubMed] [Google Scholar]
- 7.Yuan, X. M. & Li, W. (2003) Ann. Med. 35, 578-591. [DOI] [PubMed] [Google Scholar]
- 8.Kruszewski, M. (2004) Acta Biochim. Pol. 51, 471-480. [PubMed] [Google Scholar]
- 9.Stadtman, E. R. & Berlett, B. S. (1991) J. Biol. Chem. 266, 17201-17211. [PubMed] [Google Scholar]
- 10.Berlett, B. S., Chock, P. B., Yim, M. B. & Stadtman, E. R. (1990) Proc. Natl. Acad. Sci. USA 87, 389-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yim, M. B., Berlett, B. S., Chock, P. B. & Stadtman, E. R. (1990) Proc. Natl. Acad. Sci. USA 87, 394-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stadtman, E. R. & Berlett, B. S. (1989) in Oxygen Radicals in Biology and Medicine, eds. Simic, M. G., Ward, J. F. & Taylor, K. A. (Plenum, New York), pp. 131-136.
- 13.Hatch, F. T. (1968) Adv. Lipid Res. 6, 1-68. [PubMed] [Google Scholar]
- 14.Fukunaga, K., Yoshida, M. & Nakazono, N. (1998) Biomed. Chromatogr. 12, 300-303. [DOI] [PubMed] [Google Scholar]
- 15.Levine, R. L., Williams, J. A., Stadtman, E. R. & Shacter, E. (1994) Methods Enzymol. 233, 346-357. [DOI] [PubMed] [Google Scholar]
- 16.Levine, R. L., Mosoni, L., Berlett, B. S. & Stadtman, E. R. (1996) Proc. Natl. Acad. Sci. USA 93, 15036-15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fliss, H., Weissbach, H. & Brot, N. (1983) Proc. Natl. Acad. Sci. USA 80, 7160-7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine, R. L. (1983) J. Biol. Chem. 258, 11823-11827. [PubMed] [Google Scholar]
- 19.Arai, H., Terao, J., Abdalla, D. S., Suzuki, T. & Takama, K. (1996) Free Radical Biol. Med. 20, 365-371. [DOI] [PubMed] [Google Scholar]
- 20.Zhang, H., Joseph, J., Gurney, M., Becker, D. & Kalyanaraman, B. (2002) J. Biol. Chem. 277, 1013-1020. [DOI] [PubMed] [Google Scholar]
- 21.Liochev, S. I. & Fridovich, I. (2002) J. Biol. Chem. 277, 34674-34678. [DOI] [PubMed] [Google Scholar]
- 22.Sankarapandi, S. & Zweier, J. L. (1999) J. Biol. Chem. 274, 1226-1232. [DOI] [PubMed] [Google Scholar]
- 23.Elam, J. S., Malek, K., Rodriguez, J. A., Doucette, P. A., Taylor, A. B., Hayward, L. J., Cabelli, D. E., Valentine, J. S. & Hart, P. J. (2003) J. Biol. Chem. 278, 21032-21039. [DOI] [PubMed] [Google Scholar]
- 24.Lymar, S. V., Jiang, Q. & Hurst, J. K. (1996) Biochemistry 35, 7855-7861. [DOI] [PubMed] [Google Scholar]
- 25.Tien, M., Berlett, B. S., Levine, R. L., Chock, P. B. & Stadtman, E. R. (1999) Proc. Natl. Acad. Sci. USA 96, 7809-7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas, S. R., Davies, M. J. & Stocker, R. (1998) Chem. Res. Toxicol. 11, 484-494. [DOI] [PubMed] [Google Scholar]
- 27.Ferroni, F., Maccaglia, A., Pietraforte, D., Turco, L. & Minetti, M. (2004) J. Agric. Food Chem. 52, 2866-2874. [DOI] [PubMed] [Google Scholar]
- 28.Halliwell, B. & Gutteridge, J. M. C. (1999) Free Radicals in Biology and Medicine (Oxford Univ. Press, New York).
- 29.Tien, M., Morehouse, L. A., Bucher, J. R. & Aust, S. D. (1982) Arch. Biochem. Biophys. 218, 450-458. [DOI] [PubMed] [Google Scholar]
- 30.Braughler, J. M., Chase, R. L. & Pregenzer, J. F. (1987) Biochim. Biophys. Acta 921, 457-464. [DOI] [PubMed] [Google Scholar]
- 31.Gutteridge, J. M., Richmond, R. & Halliwell, B. (1979) Biochem. J. 184, 469-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samocha-Bonet, D., Gal, S., Schnitzer, E., Lichtenberg, D. & Pinchuk, I. (2004) Free Radical Res. 38, 1173-1181. [DOI] [PubMed] [Google Scholar]
- 33.Schnitzer, E., Pinchuk, I., Bor, A., Fainaru, M. & Lichtenberg, D. (1997) Biochim. Biophys. Acta 1344, 300-311. [DOI] [PubMed] [Google Scholar]
- 34.Bourdon, E., Loreau, N. & Blache, D. (1999) FASEB J. 13, 233-244. [DOI] [PubMed] [Google Scholar]
- 35.Burcham, P. L. & Kuhan, Y. T. (1996) Biochem. Biophys. Res. Commun. 220, 996-1001. [DOI] [PubMed] [Google Scholar]
- 36.Jacob, H. S. (1994) Perspect. Biol. Med. 38, 114-124. [DOI] [PubMed] [Google Scholar]
- 37.Grinshtein, N., Bamm, V. V., Tsemakhovich, V. A. & Shaklai, N. (2003) Biochemistry 42, 6977-6985. [DOI] [PubMed] [Google Scholar]
- 38.Miller, Y. I., Felikman, Y. & Shaklai, N. (1996) Arch. Biochem. Biophys. 326, 252-260. [DOI] [PubMed] [Google Scholar]
- 39.Miller, Y. I. & Shaklai, N. (1994) Biochem. Mol. Biol. Int. 34, 1121-1129. [PubMed] [Google Scholar]
- 40.Ziouzenkova, O., Asatryan, L., Akmal, M., Tetta, C., Wratten, M. L., Loseto-Wich, G., Jurgens, G., Heinecke, J. & Sevanian, A. (1999) J. Biol. Chem. 274, 18916-18924. [DOI] [PubMed] [Google Scholar]
- 41.Camejo, G., Halberg, C., Manschik-Lundin, A., Hurt-Camejo, E., Rosengren, B., Olsson, H., Hansson, G. I., Forsberg, G. B. & Ylhen, B. (1998) J. Lipid Res. 39, 755-766. [PubMed] [Google Scholar]
- 42.Richardson, D. E., Regino, C. A., Yao, H. & Johnson, J. V. (2003) Free Radical Biol. Med. 35, 1538-1550. [DOI] [PubMed] [Google Scholar]