Abstract
Ascorbic acid (Asc) is the most abundant antioxidant in plants and serves as a major contributor to the cell redox state. Exposure to environmental ozone can cause significant damage to plants by imposing conditions of oxidative stress. We examined whether increasing the level of Asc through enhanced Asc recycling would limit the deleterious effects of environmental oxidative stress. Plants overexpressing dehydroascorbate reductase (DHAR), which results in an increase in the endogenous level of Asc, were exposed to acute or chronic levels of ozone. DHAR-overexpressing plants had a lower oxidative load, a lower level of oxidative-related enzyme activities, a higher level of chlorophyll, and a higher level of photosynthetic activity 24 h following an acute exposure (2 h) to 200 ppb ozone than control plants, despite exhibiting a larger stomatal area. Reducing the size of the Asc pool size through suppression of DHAR expression had the opposite effect. Following a chronic exposure (30 d) to 100 ppb ozone, plants with a larger Asc pool size maintained a larger stomatal area and a higher oxidative load, but retained a higher level of photosynthetic activity than control plants, whereas plants suppressed for DHAR had a substantially reduced stomatal area, but also a substantially lower level of photosynthetic activity. Together, these data indicate that, despite a reduced ability to respond to ozone through stomatal closure, increasing the level of Asc through enhanced Asc recycling provided greater protection against oxidative damage than reducing stomatal area.
Despite its essential role in supporting life, oxygen can be highly damaging. In the chloroplast, excess light can result in the production of reactive oxygen species (ROS), e.g. reduction of O2 to superoxide that can occur in PSII or PSI by electrons derived from PSII. O2− is then disproportionated by superoxide dismutase (SOD) to O2 and H2O2. The H2O2 is further reduced to H2O by ascorbate peroxidase (APX) in the water-water cycle (Asada, 2000). This cycle serves to maintain electron flow through the photosystems. However, exposure to many abiotic stresses, including cold, drought, or high light, can exacerbate ROS production by creating conditions of light stress at lower photon flux density. H2O2 inactivates APX within seconds if ascorbic acid (Asc) recycling is impaired (Miyake and Asada, 1996). H2O2 can also inhibit CO2 assimilation by inhibiting several Calvin cycle enzymes (Asada, 1999). ROS can also be generated in response to exposure to pollutants, such as ozone (Grimes et al., 1983; Mudd, 1997; Schraudner et al., 1998), which can serve as signaling intermediates in guard cells to promote stomatal closure (Pei et al., 2000; Zhang et al., 2001) or, following a severe exposure, can damage cell membranes or even induce programmed cell death (Koch et al., 2000; Overmyer et al., 2000; Rao et al., 2000; Loreto et al., 2001; Pasqualini et al., 2002, 2003).
Asc is the most abundant antioxidant in plants and serves as the major contributor to the cell redox state (Smirnoff, 2000). The role that Asc plays in responding to oxidative stress has been shown with Arabidopsis (Arabidopsis thaliana) vtc mutants that are impaired in Asc biosynthesis (Conklin et al., 1999, 2000). vtc1 is defective in GDP-Man pyrophosphorylase, accumulating only 25% to 30% of the wild-type level (Conklin et al., 1999). vtc1 plants exhibit a moderately slower growth rate under normal growth conditions and are hypersensitive to ozone, sulfur dioxide, or UVB light (Conklin et al., 1996; Veljovic-Jovanovic et al., 2001), suggesting that much of the Asc in plants is essential to respond to conditions of oxidative stress. vtc2 mutants experience chronic photooxidative stress in high light and photobleach when transferred from low to high light accompanied by increased lipid peroxidation and photoinhibition (Muller-Moule et al., 2003, 2004).
Once used, Asc is oxidized to the monodehydroascorbate radical, which is reduced to Asc by monodehydroascorbate reductase (MDAR) or disproportionates to Asc and dehydroascorbate (DHA). DHA undergoes irreversible hydrolysis to 2,3-diketogulonic acid or is reduced to Asc, a reaction catalyzed by dehydroascorbate reductase (DHAR). Thus, DHAR and MDAR allow the plant to recycle oxidized Asc, thereby recapturing it before it is lost. DHAR and MDAR would be expected to be important under conditions of oxidative stress where the demand for Asc greatly increases. The importance of DHAR in preventing photoinhibition was shown in the DHAR-depleted tropical fig, which exhibits high light sensitivity (i.e. photobleaching; Yamasaki et al., 1999).
Changes in cytosolic DHAR expression affect the Asc redox state: DHAR overexpression increases the Asc pool size as well as the Asc redox state (Kwon et al., 2001; Chen et al., 2003), consistent with the function of DHAR in converting DHA to Asc. DHAR-silenced plants exhibit an increase in DHA without an increase in Asc, resulting in a substantial decrease in the Asc redox state (Chen and Gallie, 2004). Similar changes in apoplastic Asc were observed (Chen and Gallie, 2004), demonstrating that the level of cytosolic DHAR serves to regulate Asc both inside and outside the cell.
In addition to its role in photosynthesis, Asc is involved in controlling stomatal movement (Chen and Gallie, 2004). Stomatal closure occurs in response to water stress through the action of abscisic acid (ABA), which causes an increase in cytosolic Ca2+ concentration through H2O2-activated Ca2+ channels, and from release from intracellular stores (Price et al., 1994; McAinsh et al., 1996; Grabov and Blatt, 1998; Guan et al., 2000; Hamilton et al., 2000; MacRobbie, 2000; Pei et al., 2000; Zhang et al., 2001; Kohler and Blatt, 2002). ABA causes an increase in H2O2 production, which acts as a signaling intermediate in guard cells to promote stomatal closure (Price et al., 1994; McAinsh et al., 1996; Pei et al., 2000; Murata et al., 2001; Schroeder et al., 2001a, 2001b; Zhang et al., 2001; Chen et al., 2003).
Plants in which DHAR expression was increased (DHAR-OX) exhibited an increase in guard cell Asc, whereas suppression or knockdown of DHAR expression (DHAR-KD) led to the opposite effect (Chen and Gallie, 2004). DHAR-OX guard cells also exhibited reduced levels of H2O2, whereas those from DHAR-KD plants had an elevated level of H2O2. DHAR-OX plants exhibited an increase in stomatal area, both in the percentage of stomata that were open and in the degree of their openness under well-watered conditions during normal stomatal closure that occurs during the afternoon relative to control leaves (Chen and Gallie, 2004). Increasing the Asc redox state resulted in an increase in transpiration rate and stomatal conductance under normal growth conditions. DHAR-OX guard cells were less responsive to H2O2 or ABA signaling and the plants exhibited enhanced water loss following the imposition of drought conditions (Chen and Gallie, 2004). Decreasing the Asc redox state by repressing DHAR expression enhanced stomatal closure under normal growth conditions and following a water stress. These observations suggest that Asc plays an important role in controlling H2O2-mediated stomatal closure. Enhanced tolerance to H2O2 and other abiotic stresses was observed in tobacco (Nicotiana tabacum) expressing a human DHAR (Kwon et al., 2003).
Exposure to ozone results in foliar injury, impaired photosynthesis, reduced growth and yield, and an accelerated onset of senescence in plants (Heggestad and Middleton, 1959; Heath, 1980, 1987; Sakaki et al., 1983; Heagle, 1989; Langebartels et al., 1990; Sen Gupta et al., 1991; Ballach et al., 1992). Sensitivity to ozone is inversely correlated with the level of foliar Asc (Freebairn, 1960; Freebairn and Taylor, 1960; Menser, 1964; Lee et al., 1984; Lee, 1991; Luwe et al., 1993; Machler et al., 1995; Conklin et al., 1996) and the level of Asc in the apoplast, and the rate of its regeneration contributes significantly to the detoxification of invading ozone (Castillo and Greppin, 1988; Luwe and Heber, 1995; Polle et al., 1995; Conklin et al., 1996; Moldau et al., 1997, 1998; Lyons et al., 1999; Turcsanyi et al., 2000).
Plants can limit the damage caused by exposure to oxidizing environmental pollutants such as ozone by avoidance or tolerance (Taylor, 1978). Avoidance involves limiting entry of ozone to the interior of the leaf through stomatal closure. Following exposure to ozone, ROS are first observed in guard cell chloroplasts and membranes and later spread to neighboring cells (Joo et al., 2005). The increase in the level of ROS in guard cells limits further diffusion of ozone into the leaf by promoting stomatal closure. Following entry through open stomata, ozone rapidly degrades into hydroxyl radicals and other ROS that can be converted to H2O2 (Grimes et al., 1983; Mudd, 1997). Tolerance includes the detoxification of ozone that does diffuse into the leaf interior, either through its direct chemical reaction with apoplastic Asc or, if it enters the cellular environment and is converted to H2O2, enzymatically by APX. As the level of DHAR expression determines the efficiency of Asc recycling (Chen et al., 2003; Chen and Gallie, 2004), increasing the efficiency of Asc recycling in leaves by increasing DHAR expression would be expected to decrease guard cell responsiveness to ozone, resulting in a larger stomatal area and increased ozone diffusion, while increasing the ability of leaf cells to detoxify the invading ozone. Conversely, reducing the efficiency of Asc recycling through the suppression of DHAR expression would be expected to increase guard cell responsiveness to ozone and thus reduce ozone diffusion into the leaf interior by reducing stomatal area, while the reduction in Asc recycling would be predicted to impair the ability of leaf cells to detoxify any ozone that did invade the leaf interior.
Using plants in which the efficiency of Asc recycling was increased or reduced, we examined whether altering guard cell responsiveness or the foliar level of Asc itself confers greater protection to the oxidative stress imposed by an acute or chronic exposure to ozone. We observed that DHAR-OX guard cells were less responsive to ozone, resulting in a larger stomatal area after the ozone treatment than control guard cells. DHAR-OX leaves also maintained a higher level of Asc than the control following ozone treatment. As a consequence, DHAR-OX plants had a lower oxidative load, a lower level of oxidative-related enzyme activities, greater chlorophyll (Chl) a content, and a higher level of photosynthetic activity following an acute exposure to ozone despite exhibiting a larger open stomatal area. Reducing the size of the Asc pool size through suppression of DHAR expression had the opposite effect. Following a chronic exposure to ozone, plants with a larger Asc pool size had a larger stomatal area and a higher oxidative load, but retained a higher level of photosynthetic activity than control plants, whereas DHAR-KD plants had a substantially reduced stomatal area, but also a substantially lower level of photosynthetic activity. Together, these data indicate that, despite a reduced ability to respond to ozone through stomatal closure, enhancing Asc recycling in DHAR-OX leaves provides greater protection against oxidative damage than reducing total stomatal area.
RESULTS
Plants Overexpressing DHAR Maintain a Larger Asc Pool Size following Exposure to Ozone
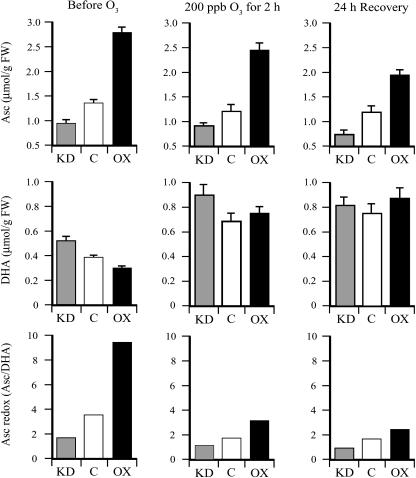
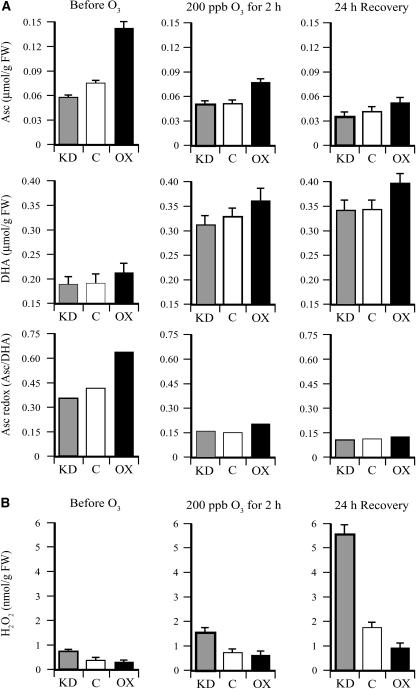
Transgenic DHAR-OX and DHAR-KD tobacco lines have been described previously (Chen et al., 2003; Chen and Gallie, 2004). DHAR-OX leaves, in which DHAR expression was increased up to 13-fold, exhibited a 2.1-fold increase in the level of Asc and a 25% reduction in the level of DHA, resulting in an increase in the Asc redox state (i.e. the Asc/DHA ratio) from 3.4 in control leaves to 9.3 in DHAR-OX leaves (Fig. 1). DHAR-KD leaves, in which DHAR expression was suppressed up to 77%, exhibited a 29% reduction in the level of Asc and a 32% increase in the level of DHA, resulting in a decrease in the Asc redox state from 3.4 in control leaves to 1.8 in DHAR-KD leaves. Following an acute exposure (2 h) to 200 ppb ozone, the foliar level of Asc was reduced slightly, whereas the level of DHA increased substantially in DHAR-OX, DHAR-KD, and control plants, resulting in a decrease in the Asc redox state (Fig. 1). A further reduction in Asc was observed following a 24-h period of recovery. Despite a disproportionate reduction in the redox state in DHAR-OX leaves, the Asc pool size and redox state in these leaves remained higher than in control or DHAR-KD leaves.
Figure 1.
DHAR-OX plants maintain a higher foliar level of Asc following exposure to an acute episode of ozone. The levels of Asc and DHA were measured from the first fully expanded leaf of three replicate control (C), DHAR-OX (OX), and DHAR-KD (KD) tobacco collected prior to, immediately after, and 24 h following a 2-h exposure to 200 ppb ozone. The Asc redox state determined by [Asc]/[DHA] is also shown.
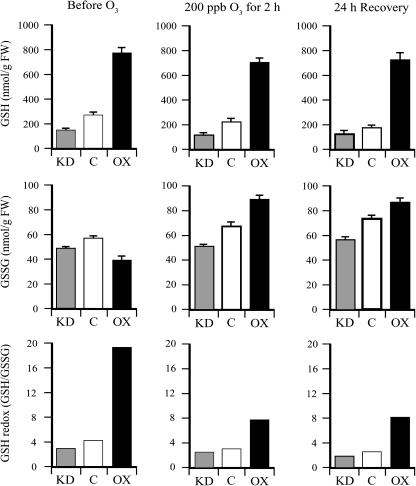
In addition to Asc, changes in DHAR expression affect the glutathione (GSH) pool size and redox state (Chen et al., 2003; Chen and Gallie, 2004). DHAR-OX leaves exhibited a 2.8-fold increase in the level of GSH and a 32% reduction in the level of oxidized glutathione (GSSG), resulting in an increase in the GSH redox state (i.e. the GSH/GSSG ratio) from 4.6 in control leaves to 19 in DHAR-OX leaves (Fig. 2). DHAR-KD leaves exhibited a 45% and 20% reduction in the level of GSH and GSSG, respectively, resulting in a decrease in the GSH redox state from 4.6 in control leaves to 3.1 in DHAR-KD leaves. As with Asc, an acute exposure to ozone resulted in a slight reduction in GSH, an increase in GSSG, and a reduction in the GSH redox state in DHAR-OX, DHAR-KD, and control plants (Fig. 2). Little additional change was observed following a 24-h period of recovery.
Figure 2.
DHAR-OX plants maintain a higher foliar level of GSH following exposure to an acute episode of ozone. The levels of GSH and GSSG were measured from the first fully expanded leaf of control (C), DHAR-OX (OX), and DHAR-KD (KD) tobacco collected prior to, immediately after, and 24 h following a 2-h exposure to 200 ppb ozone. The GSH redox state determined by [GSH]/[GSSG] is also shown.
DHAR-KD Plants Undergo Greater Damage following Exposure to Ozone
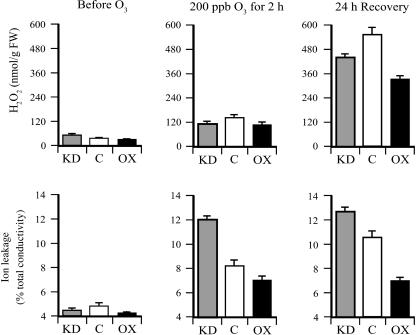
To determine whether the foliar level of H2O2 increased following an acute exposure to ozone, the amount of H2O2 was measured in leaves of DHAR-OX, DHAR-KD, and control plants before and after ozone treatment. Prior to the treatment, the foliar level of H2O2 in all lines was low when measured in early morning, with the level of H2O2 significantly lower in DHAR-OX leaves than in DHAR-KD leaves (P < 0.05; n = 3) and significantly higher in DHAR-KD leaves than in control leaves (P < 0.05; n = 3). Immediately following the 2-h exposure to 200 ppb ozone, the foliar level of H2O2 increased approximately 4-fold in control and DHAR-OX leaves, but only 2.3-fold in DHAR-KD leaves (Fig. 3), consistent with the notion that ozone diffusion into leaves of the latter was more limited than in control or DHAR-OX leaves. As a result, the level of H2O2 in DHAR-KD leaves was not significantly different from that in DHAR-OX or control leaves, although the level in control leaves was significantly higher than in DHAR-OX leaves (P < 0.05; n = 3). Following a 24-h recovery, the level of H2O2 increased substantially in all three lines, but was lower in DHAR-OX leaves than in the control (Fig. 3), consistent with an increased ability to detoxify invading ozone. Similarly, a lower level of H2O2 was observed in DHAR-KD leaves relative to the control, consistent with an increased ability to limit ozone diffusion.
Figure 3.
DHAR-OX plants exhibit less damage following exposure to an acute episode of ozone. The level of H2O2 was measured from the first fully expanded leaf of control (C), DHAR-OX (OX), and DHAR-KD (KD) tobacco collected prior to, immediately after, and 24 h following a 2-h exposure to 200 ppb ozone. Damage from ozone exposure was measured as ion leakage from fully expanded leaves.
The degree to which exposure to ozone resulted in membrane damage was assayed by measuring ion leakage. Acute ozone exposure resulted in a substantial increase in ion leakage in all three lines, but was highest in DHAR-KD leaves and lowest in DHAR-OX leaves, whereas the extent of ion leakage in control leaves was between these two extremes. These data indicate that DHAR-KD leaves sustained the highest level of membrane damage, whereas DHAR-OX leaves sustained the least. It is interesting to note that a greater degree of membrane damage was observed in DHAR-KD plants relative to the control despite the fact that their level of H2O2 was lower than in control leaves. This suggests that, although DHAR-KD plants are better in limiting ozone diffusion, they may be more vulnerable to any ozone that does enter because of their reduced ability to detoxify ozone.
The Level of Asc Determines the Extent of Guard Cell Responsiveness to Ozone
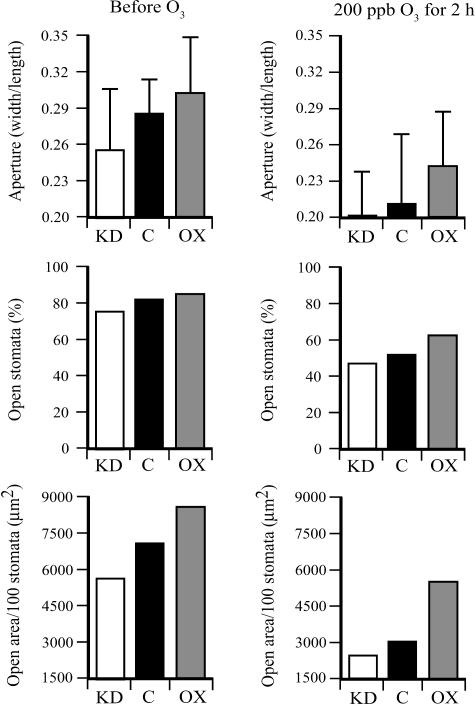
DHAR-OX guard cells were shown to be less responsive to ABA-induced stomatal closure than control guard cells (Chen and Gallie, 2004). To examine whether the level of Asc had a similar effect on guard cell responsiveness following exposure to ozone, the stomatal aperture and the percentage of stomata that were open were measured before and immediately after ozone treatment. Prior to the ozone treatment, the average stomatal aperture, the percent of stomata that were open, and the total stomatal area as measured in the morning were larger in DHAR-OX leaves and smaller in DHAR-KD leaves relative to the control (Fig. 4). These differences are in good agreement with previous measurements, where only moderate differences are observed in the morning when most stomata are open (Chen and Gallie, 2004). Exposure to 2 h of 200 ppb ozone was sufficient to induce stomatal closure in all three lines. However, DHAR-OX guard cells were less responsive to ozone-induced closure than were DHAR-KD guard cells in that ozone treatment induced a 1.7-fold reduction in stomatal area in DHAR-OX leaves, whereas a 2.3-fold reduction in stomatal area was observed in DHAR-KD leaves (Fig. 4).
Figure 4.
The level of DHAR expression determines guard cell responsiveness in response to an acute exposure of ozone. The stomatal aperture (width/length), percent of stomata that were open (defined as having a width greater than 1 μm), and the total stomatal area per 100 stomata were determined from the abaxial epidermis of fully expanded control (C), DHAR-OX (OX), and DHAR-KD (KD) tobacco leaves prior to, immediately after, and 24 h following a 2-h exposure to 200 ppb ozone. The width and length of at least 30 stomatal apertures were measured and used to determine the stomatal aperture (width/length) with the sd indicated. The total stomatal open area was calculated from the average stomatal aperture area and percent of stomata that were open and reported as the total area (μm2) per 100 stomata.
To examine whether the differences in guard cell response following ozone treatment affected the level of apoplastic Asc and Asc redox state, each was measured from the apoplastic fluid collected from leaves of the three lines before and after exposure to ozone. The level of Asc and the Asc redox state in the apoplast is significantly lower than in the cytosol (Vanacker et al., 1998; Cordoba-Pedregosa et al., 2003a, 2003b; Chen and Gallie, 2004; de Pinto and De Gara, 2004). The level of apoplastic Asc and the Asc redox state were significantly higher in DHAR-OX leaves and significantly lower in DHAR-KD leaves relative to the control prior to ozone treatment (P < 0.005; n = 3 and P < 0.05; n = 3, respectively; Fig. 5A). A reduction in apoplastic Asc and a substantial increase in apoplastic DHA were observed immediately following exposure to ozone. The level of Asc was significantly higher in DHAR-OX leaves than in control (P < 0.05; n = 3) or DHAR-KD (P < 0.05; n = 3) leaves, but not significantly different between DHAR-KD and control leaves. The level of apoplastic Asc decreased further during the 24-h recovery, where a significant difference in Asc was observed only between DHAR-OX and DHAR-KD leaves (P = 0.05; n = 3). The apoplastic Asc redox state decreased to a greater extent in DHAR-OX leaves than in either DHAR-KD or control leaves as a consequence of more extensive oxidation of Asc into DHA.
Figure 5.
DHAR-OX plants maintain a higher apoplastic level of Asc following exposure to an acute episode of ozone. A, The levels of Asc and DHA were measured from the apoplastic fluid of the first fully expanded leaf of control (C), DHAR-OX (OX), and DHAR-KD (KD) tobacco collected prior to, immediately after, and 24 h following a 2-h exposure to 200 ppb ozone. The Asc redox state determined by [Asc]/[DHA] is also shown. B, The level of H2O2 was measured from the apoplastic fluid of the first fully expanded leaf collected prior to, immediately after, and 24 h following the ozone treatment.
An inverse correlation between the level of apoplastic Asc and Asc redox state levels with apoplastic H2O2 was also observed. The level of apoplastic H2O2 was low in the three lines prior to ozone treatment (Fig. 5B), but increased immediately following exposure to ozone and increased further, i.e. the oxidative burst previously described following ozone exposure (Schraudner et al., 1998; Wohlgemuth et al., 2002; Pasqualini et al., 2003), during the recovery period. The level of apoplastic H2O2 was higher in DHAR-KD leaves and lower in DHAR-OX leaves relative to the control before and after ozone treatment, but the level increased disproportionately in DHAR-KD leaves and increased least in DHAR-OX leaves (Fig. 5B). Together, these data suggest that the increased amount of Asc in the apoplast was used to detoxify the invading ozone. They also indicate that the ozone-induced reduction in stomatal area in DHAR-KD leaves was not sufficient to prevent an increase in apoplastic H2O2 and that DHAR-KD leaves were less able to respond to the oxidative burst of H2O2 during recovery than DHAR-OX leaves.
Ozone Induction of Oxidative Stress-Related Enzyme Activities Is Altered following Changes in DHAR Expression
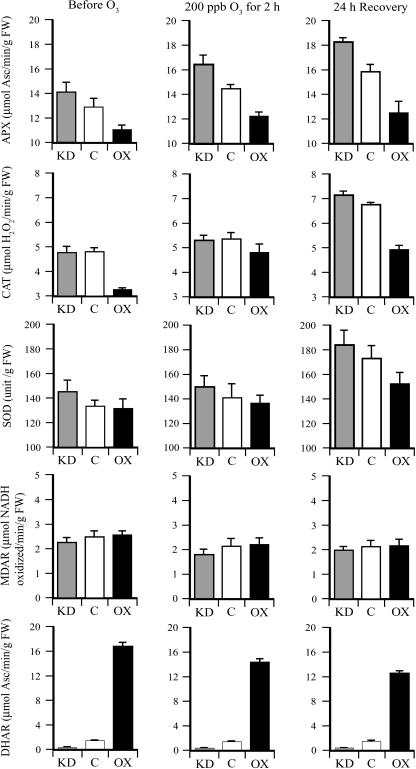
Exposure to ozone induces activity or expression of oxidative stress-related enzymes including APX, catalase (CAT), and SOD (Tanaka et al., 1985; Mehlhorn et al., 1990; Herouart et al., 1991; Bowler et al., 1992; Sharma and Davis, 1994; Willekens et al., 1994). To examine the effect of Asc on the ozone induction of oxidative stress-related enzyme activities, APX, CAT, and SOD activities were measured in leaves of the three lines before and after exposure to ozone. APX activity was higher in DHAR-KD leaves and lower in DHAR-OX leaves relative to the control before and after ozone treatment. Exposure to ozone induced a higher level of APX activity in all lines, including during the 24-h recovery period (Fig. 6). A similar induction in CAT activity by ozone was observed, but a lower level of activity was observed in DHAR-OX leaves relative to the control or DHAR-KD leaves, particularly following recovery (Fig. 6). Induction of SOD activity by ozone was observed only after the 24-h recovery period following exposure to ozone, but SOD activity was higher in DHAR-KD leaves and lower in DHAR-OX leaves relative to the control immediately after and 24 h following the ozone treatment (Fig. 6). No induction of MDAR activity was observed following ozone treatment (Fig. 6). Ozone treatment also reduced DHAR activity, but the activity remained higher in DHAR-OX leaves and lower in DHAR-KD leaves relative to the control (Fig. 6).
Figure 6.
Foliar levels of APX, CAT, SOD, and DHAR activities in DHAR-OX and DHAR-KD following exposure to an acute episode of ozone. APX, CAT, SOD, and DHAR activities were measured in the first fully expanded leaf of DHAR-OX and DHAR-KD tobacco collected prior to, immediately after, and 24 h following a 2-h exposure to 200 ppb ozone. Enzyme activities were assayed in three independent replicates of leaves pooled from three independent plants and the average and sd reported.
Greater Chl a Content Is Maintained in DHAR-OX Leaves following Ozone Exposure
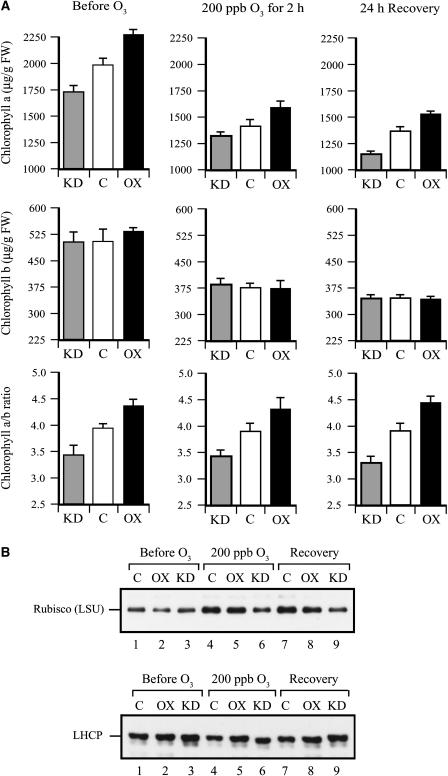
Exposure to ozone results in a reduction in Chl content (Reich, 1983). To examine whether the level of Asc is important in protecting against an ozone-induced loss of Chl, the level of Chl a and Chl b as well as the Chl a/b ratio were measured in the three lines before and after ozone treatment. DHAR-OX leaves contained greater Chl a content and DHAR-KD leaves contained lower Chl a content than control leaves (Fig. 7A). Exposure to ozone resulted in a substantial decrease in Chl a content, but the relative differences among the three lines remained the same. Little difference in Chl b content was observed among the three lines and, although exposure to ozone also resulted in a substantial decrease in Chl b content, no significant difference in the relative level among the three lines was observed (Fig. 7A). As a consequence, the Chl a/b ratio was higher in DHAR-OX leaves and lower in DHAR-KD leaves relative to the control and, despite the ozone-induced reduction in Chl a and Chl b content, the Chl a/b ratio remained unaffected by the ozone treatment. These data demonstrate that Chl a is protected against ozone by a higher Asc pool size.
Figure 7.
DHAR-OX plants maintain greater Chl a content following exposure to an acute episode of ozone. A, The levels of Chl a and Chl b were measured from the first fully expanded leaf of control (C), DHAR-OX (OX), and DHAR-KD (KD) tobacco collected prior to, immediately after, and 24 h following a 2-h exposure to 200 ppb ozone. The Chl a/b ratio determined by [Chl a]/[Chl b] is also shown. B, The levels of rbcL (LSU) and LHCP in fully expanded leaves were determined on an equal fresh weight basis by western analysis.
Exposure to ozone can result in a loss of Rubisco expression or activity (Pell and Pearson, 1983; Dann and Pell, 1989; Pell et al., 1990, 1997; Held et al., 1991; Nie et al., 1993; Bahl and Kahl, 1995; Conklin and Last, 1995; Rao et al., 1995). Therefore, the level of Rubisco, as measured by the amount of the rbcL, and the level of light-harvesting complex protein (LHCP) were measured. Similar levels of rbcL were observed among the three lines prior to the ozone treatment (Fig. 7B). In contrast, a lower level was observed in DHAR-KD leaves immediately following ozone treatment and after 24 h of recovery relative to that in DHAR-OX and control leaves. No consistent difference in LHCP was observed among the three lines before and after ozone treatment.
DHAR-OX Plants Maintain a Higher Rate of CO2 Assimilation following Exposure to Ozone
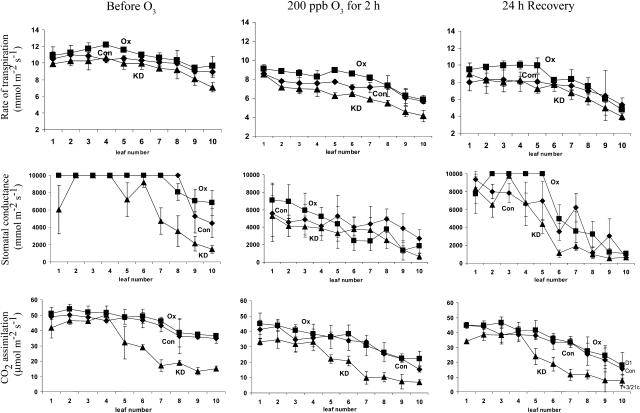
DHAR-OX leaves exhibited a slightly higher rate of CO2 assimilation and DHAR-KD leaves a lower rate of CO2 assimilation compared to the control, especially in the older leaves (Fig. 8). As exposure to ozone can inhibit photosynthesis (Runeckles and Chevone, 1992; Pell et al., 1997), whether an increase in foliar Asc could protect photosynthesis against ozone to a greater extent despite a reduction in guard cell responsiveness was examined by measuring CO2 assimilation in leaves of the three lines immediately and 24 h after ozone treatment. Following ozone exposure, a substantial decrease in stomatal conductance was observed in all three lines, but increased again following 24 h of recovery (Fig. 8). The rate of CO2 assimilation decreased in all three lines immediately following exposure to ozone and exhibited only a slight improvement by 24 h of recovery (Fig. 8). Nevertheless, DHAR-OX leaves maintained a higher rate of CO2 assimilation and DHAR-KD leaves exhibited a lower rate of CO2 assimilation relative to control leaves (Fig. 8). This is despite a greater rate of transpiration and stomatal conductance in DHAR-OX leaves relative to control or DHAR-KD leaves that would have permitted greater ozone diffusion into the leaf interior (Fig. 8). These data indicate that increasing foliar Asc levels maintains photosynthesis against invading ozone to a greater extent despite a reduction in guard cell responsiveness.
Figure 8.
DHAR-OX plants maintain a higher rate of photosynthesis following exposure to an acute episode of ozone. In situ rates of transpiration (top), stomatal conductance (middle), and CO2 assimilation (bottom) were measured in control (Con), DHAR-OX (OX), and DHAR-KD (KD) leaves prior to, immediately after, and 24 h following a 2-h exposure to 200 ppb ozone. Measurements were made in every other leaf in three independent replicates of leaves pooled from three independent plants and the average and sd reported. Leaf 1 is the youngest leaf measured; leaf 10 is the oldest.
Increasing Foliar Asc Maintains a Higher Level of Photosynthetic Activity during Chronic Exposure to Ozone
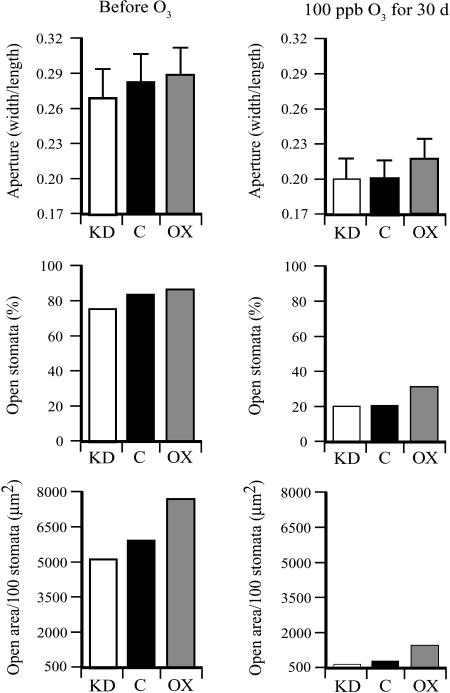
To examine whether increasing the foliar level of Asc conferred greater tolerance to ozone under chronic exposure conditions, DHAR-OX, DHAR-KD, and control plants were exposed to 100 ppb ozone for 4 h every morning for 30 d. Prior to the ozone treatment, the stomatal area in DHAR-OX leaves in the morning was larger and that of DHAR-KD leaves smaller than the control (Fig. 9), as observed in Figure 4. Following a chronic ozone treatment, stomatal closure was induced in all three lines to a significantly greater extent than that observed after the acute exposure to ozone. However, as with the acute exposure, DHAR-OX guard cells remained less responsive to ozone even after the 30-d treatment than DHAR-KD or control guard cells, whereas guard cells in DHAR-KD leaves were more responsive to ozone (Fig. 9).
Figure 9.
The level of DHAR expression determines guard cell responsiveness in response to a chronic exposure to ozone. The stomatal aperture (width/length), percent of stomata that were open (defined as having a width greater than 1 μm), and the total stomatal area per 100 stomata were determined from the abaxial epidermis of fully expanded control (C), DHAR-OX (OX), and DHAR-KD (KD) tobacco leaves prior to and after 30 d of exposure to 100 ppb ozone for 4 h/d. The width and length of at least 30 stomatal apertures were measured and used to determine the stomatal aperture (width/length) with the sd indicated. The total stomatal open area was calculated from the average stomatal aperture area and percent of stomata that were open, and reported as the total area (μm2) per 100 stomata.
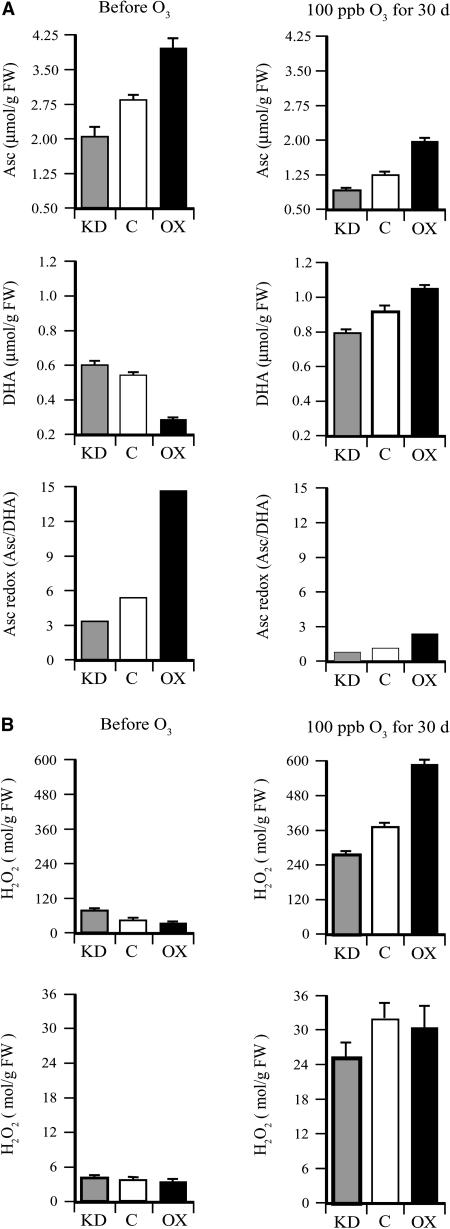
As observed in Figure 1, the level of foliar Asc and the Asc redox state were higher in DHAR-OX leaves and lower in DHAR-KD leaves relative to the control prior to the chronic ozone treatment (Fig. 10A). The level of foliar Asc decreased and the level of DHA increased in all three lines following the ozone treatment and, as observed following an acute exposure, the Asc redox state decreased to a greater extent in DHAR-OX leaves than in DHAR-KD leaves, but remained higher after the 30-d treatment than it did in DHAR-KD or control leaves, whereas the redox state of DHAR-KD leaves was lower than that in control leaves.
Figure 10.
DHAR-OX plants maintain a higher foliar level of Asc following exposure to an acute episode of ozone. A, The levels of Asc and DHA were measured from fully expanded leaves from control (C), DHAR-OX (OX), and DHAR-KD (KD) tobacco collected prior to and after 30 d of exposure to 100 ppb ozone for 4 h/d. The Asc redox state determined by [Asc]/[DHA] is also shown. B, The level of foliar (top) and apoplastic (bottom) H2O2 was measured from fully expanded leaves collected prior to and after 30 d of exposure to 100 ppb ozone for 4 h/d.
The level of foliar H2O2 was higher in DHAR-KD leaves and lower in DHAR-OX leaves relative to the control before ozone treatment and increased substantially following the chronic ozone treatment (Fig. 10B), as observed in Figure 5B. Nevertheless, in contrast to the relative levels of foliar H2O2 induced following an acute exposure, a higher level of foliar H2O2 was observed in DHAR-OX leaves and a lower level induced in DHAR-KD leaves relative to the control following a chronic exposure to ozone (Fig. 10B). Therefore, the level of foliar H2O2 increased disproportionately in DHAR-OX leaves relative to DHAR-KD leaves. Chronic exposure to ozone also resulted in a substantial increase in apoplastic H2O2 and the level was higher in DHAR-OX leaves than in DHAR-KD leaves (Fig. 10B). These data suggest that the reduced guard cell responsiveness and the resulting larger stomatal area in DHAR-OX leaves enabled greater ozone diffusion into the leaf interior over the course of the 30-d treatment than in control or DHAR-KD leaves.
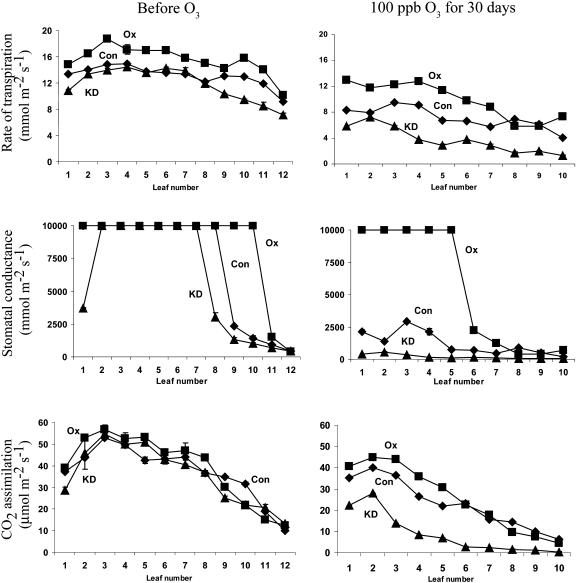
CO2 assimilation was then measured in leaves of the three lines before and after the 30-d treatment with 100 ppb ozone. Because of the length of the treatment, younger plants were used at the beginning of the experiment. As a result, the difference in the rate of CO2 assimilation among the three lines was smaller when measurements were taken in the morning. However, as with an acute exposure, DHAR-OX leaves exhibited a higher rate of CO2 assimilation in young and fully expanded leaves than in the same leaves in control plants following a chronic exposure to ozone, whereas DHAR-KD leaves exhibited a substantially reduced rate of CO2 assimilation (Fig. 11). The higher rate of CO2 assimilation in young and fully expanded DHAR-OX leaves occurred despite the greater rate of transpiration and stomatal conductance in DHAR-OX leaves relative to control or DHAR-KD leaves that permits greater diffusion of ozone into the leaf and higher production of H2O2 (Fig. 11). In contrast, a reduced rate of CO2 assimilation in DHAR-KD leaves following a chronic exposure to ozone was observed that may be due to a lower rate of transpiration and stomatal conductance as a consequence of the increased responsiveness of their guard cells. These data indicate that increasing foliar Asc levels maintain a higher rate of photosynthesis when exposed to chronic ozone conditions or acute episodes of ozone.
Figure 11.
DHAR-OX plants maintain a higher rate of photosynthesis following a chronic exposure to ozone. In situ rates of transpiration (top), stomatal conductance (middle), and CO2 assimilation (bottom) were measured in control (Con), DHAR-OX (OX), and DHAR-KD (KD) leaves prior to and after 30 d of exposure to 100 ppb ozone for 4 h/d. Measurements were made with three independent replicates of leaves pooled from independent plants and the average and sd reported. Leaf 1 represents the youngest expanding leaf for each measurement so that leaves at similar developmental states could be compared before and after the ozone treatment.
DISCUSSION
Plants can limit damage caused by environmental ROS either by reducing their diffusion into the leaf interior by stomatal closure (i.e. avoidance) or by detoxification if they do enter (i.e. tolerance). Overexpression of DHAR increases foliar and guard cell Asc, which reduces guard cell responsiveness to ROS such as H2O2, whereas suppression of DHAR expression has the opposite effect (Chen and Gallie, 2004). Thus, increasing foliar Asc would be expected to allow greater diffusion of ROS into the leaf interior (i.e. reduced avoidance) while increasing the ability of leaf cells to detoxify invading ROS (i.e. enhanced tolerance). In contrast, decreasing foliar Asc would limit ROS diffusion by increasing guard cell responsiveness (i.e. enhanced avoidance) while decreasing the ability of leaf cells to detoxify the ROS that does enter (i.e. reduced tolerance). Thus, we could examine the contribution that Asc in interior leaf cells versus guard cells makes to ameliorating the effect of environmental ROS.
Exposure to 200 ppb ozone for just 2 h resulted in a substantial increase in the level of foliar H2O2 in all lines tested. Twenty-four hours after ozone exposure, a substantial and further increase in the level of foliar H2O2 was observed. These observations are consistent with previous studies showing that H2O2 is first produced during ozone exposure that is followed by a second oxidative burst after the cessation of ozone exposure (Schraudner et al., 1998; Wohlgemuth et al., 2002; Pasqualini et al., 2003). A lower level of H2O2 was observed in DHAR-KD and DHAR-OX leaves relative to the control both immediately after and following 24 h of recovery. The lower level of H2O2 in DHAR-OX leaves can be understood by the elevated level of Asc able to detoxify invading ozone, whereas the lower level of H2O2 in DHAR-KD leaves may be due to the greater responsiveness of their guard cells limiting ozone diffusion. The disproportionate reduction in the Asc redox state in DHAR-OX leaves following an acute exposure to ozone observed in Figure 1 suggests that the increased amount of Asc in these leaves was used to detoxify the invading ROS. Exposure to ozone resulted in a substantial decrease in apoplastic Asc, which inversely correlated with the increase in apoplastic H2O2. The disproportionate increase in apoplastic H2O2 in DHAR-KD leaves during or following ozone exposure suggests that these plants were less able to detoxify invading ozone and thus indicates that the level of apoplastic Asc may determine the level of apoplastic H2O2 accumulation.
As reported previously (Chen et al., 2003), overexpression of DHAR resulted in an increase in the GSH pool size. Because DHAR uses GSH to reduce DHA to Asc, the increase in the GSH pool size in DHAR-overexpressing leaves may be in response to the greater demand for GSH, suggesting that the GSH pool size may be regulated in part by the demand for GSH. In addition to what may be an indirect contribution to ozone detoxification, it is also possible that GSH participates directly to detoxify ozone (Hausladen and Alscher, 1993).
Guard cells of DHAR-OX leaves were less responsive to ozone than those of DHAR-KD leaves, resulting in a larger stomatal area in ozone-treated DHAR-OX plants than in DHAR-KD plants. The higher rates of stomatal conductance and transpiration in ozone-treated DHAR-OX plants are consistent with the observed larger stomatal area. This larger stomatal area and rate of stomatal conductance should result in a higher H2O2 concentration in the leaf interior of DHAR-OX plants than in DHAR-KD leaves. The fact that DHAR-OX plants maintain a lower level of foliar and apoplastic H2O2 following acute ozone exposure, despite a larger stomatal area and higher rate of stomatal conductance, supports the conclusion that detoxification of invading ROS plays a more significant role in responding to ozone than guard cell responsiveness.
When the effect of ozone on cell membranes was measured by the degree of ion leakage, DHAR-OX leaves exhibited the lowest level of ion leakage and consequently the least damage, while DHAR-KD leaves exhibited the most damage. These observations suggest that the elevated levels of foliar and apoplastic Asc in DHAR-OX leaves provided greater protection against ozone-induced damage, whereas the reduction in stomatal area observed in DHAR-KD leaves was not sufficient to compensate for the damage caused by the ozone that did enter the leaf interior. APX, SOD, and CAT activities were induced highest in DHAR-KD leaves and lowest in DHAR-OX leaves, supporting the conclusion that DHAR-KD plants were more stressed than DHAR-OX plants.
Exposure to ozone also resulted in a reduction in Chl content, demonstrating that Chl loss is part of the damage caused by ozone exposure. Ozone resulted in a similar reduction in Chl a and Chl b content, resulting in little change to the Chl a/b ratio. As with the measurement of ozone-induced damage by ion leakage, DHAR-OX leaves maintained greater Chl a content and DHAR-KD leaves exhibited a greater loss of Chl a than control plants, again supporting the conclusion that a higher foliar Asc level provides greater protection against ozone-induced loss of Chl than increasing guard cell responsiveness.
Following a chronic exposure to ozone, some differences in the response of the lines was observed. As in the acutely exposed plants, DHAR-OX leaves maintained a higher level of Asc than DHAR-KD leaves following 30 d of exposure to 100 ppb ozone. Moreover, guard cells of DHAR-OX plants remained less responsive to ozone than those of DHAR-KD plants following a chronic exposure, resulting in a substantially larger stomatal area, observations supported by the stomatal conductance measurements. However, in contrast to acutely exposed plants, following a chronic exposure to ozone, DHAR-OX plants exhibited higher foliar and apoplastic H2O2 levels than DHAR-KD plants, suggesting that, over a long period of exposure to ozone, the larger stomatal area in DHAR-OX leaves resulted in a greater accumulation of ROS both in the apoplast and intracellularly. The lower accumulation of foliar and apoplastic H2O2 in chronically exposed DHAR-KD leaves supports the notion that increasing guard cell responsiveness and thus reducing stomatal area limits entry of ozone into the leaf interior.
In addition to its effect on guard cells, ozone exposure can result in reduced photosynthesis (Runeckles and Chevone, 1992; Pell et al., 1997). This has been suggested to occur, in part, from reduced stomatal conductance or accelerated loss of D1 protein affecting photosynthetic activity (Pell et al., 1990; Godde and Buchhold, 1992; Pino et al., 1995). However, despite their higher rate of stomatal conductance and greater accumulation of ROS following a chronic exposure to ozone, DHAR-OX leaves maintained a higher rate of CO2 assimilation than control leaves, whereas DHAR-KD leaves exhibited a reduced rate of CO2 assimilation. The reduction in photosynthetic activity in DHAR-KD leaves may have been due to reduced gas exchange (i.e. reduced stomatal conductance) and/or damage to the photosynthetic machinery as evidenced by the observed reduction in the level of Rubisco. These data indicate that, even with a higher oxidative load resulting from a larger stomatal area, the higher foliar Asc pool size in DHAR-OX leaves protects the photosynthetic machinery to a greater degree than increasing guard cell responsiveness following a chronic exposure to ozone.
Thus, plants with a larger Asc pool size, but reduced guard cell responsiveness, exhibited a lower oxidative load, reduced induction of oxidative-related enzyme activities, greater Chl a content, and a higher level of photosynthetic activity following an acute ozone exposure than plants with a reduced Asc pool size, but increased guard cell responsiveness. Following a chronic ozone exposure, plants with a larger Asc pool size exhibited a higher oxidative load, but retained a higher level of photosynthetic activity despite a larger stomatal area than plants with a reduced Asc pool size, which exhibited a lower oxidative load, but also a substantially lower level of photosynthetic activity. Together, these data indicate that, despite a reduced ability to respond to ozone through stomatal closure, a higher foliar level of Asc confers a greater degree of protection against environmental oxidative damage than increasing guard cell responsiveness.
MATERIALS AND METHODS
Plant Materials
Full-length wheat (Triticum aestivum) and tobacco (Nicotiana tabacum) DHAR cDNAs (accession nos. AY074784 and AY074787, respectively) were isolated as described previously (Chen et al., 2003). Transgenic tobacco (cv Xanthi) expressing the His-tagged wheat DHAR from the cauliflower mosaic virus 35S promoter (DHAR-OX) was generated using Agrobacterium tumefaciens as described (Chen et al., 2003). Transgenic tobacco plants in which endogenous DHAR expression was knocked down (DHAR-KD) were identified following the introduction of a tobacco DHAR construct in pBI101 as described (Chen and Gallie, 2004).
All plants were grown in 5-gallon pots with commercial soil in a greenhouse supplied with charcoal-filtered air. Test plants were treated with ozone in continuously stirred tank reactors. Ozone fumigations were performed in the morning, when stomata are fully open. Ozone was generated by passing tank oxygen through an ozonizer and by irradiation with UV light, transported to Brooks (Hatfield, PA) 5850 series mass flow controllers through a manifold and then delivered to fumigation chambers in which the plants were present. Ozone levels were continuously monitored by multiport sampling valves leading to a Dasibi ozone analyzer (model 1003-AH; Dasibi Environmental Corporation, Glendale, CA). Plants were exposed to 200 ppb for 2 h for an acute treatment or 100 ppb for 4 h every morning for 30 d for a chronic treatment. Control plants were grown in a greenhouse supplied with charcoal-filtered air. Plants used in the experiments had produced approximately 20 leaves and had not yet produced an inflorescence.
Western Analysis
Protein extracts were resolved using standard SDS-PAGE and the protein transferred to 0.22 μm polyvinylidene difluoride membrane by electroblotting. Following transfer, the polyvinylidene difluoride membranes were blocked in 5% milk, 0.01% thimerosal in Tween phosphate-buffered saline (TPBS; 0.1% Tween 20, 13.7 mm NaCl, 0.27 mm KCl, 1 mm Na2HPO4, 0.14 mm KH2PO4), followed by incubation with anti-DHAR (Chen et al., 2003), anti-Rubisco, or anti-LHCP antisera diluted typically 1:1,000 to 1:2,000 in TPBS with 1% milk for 1.5 h. The blots were then washed twice with TPBS and incubated with goat anti-rabbit horseradish peroxidase-conjugated antibodies (Southern Biotechnology Associates, Birmingham, AL ) diluted to 1:5,000 to 1:10,000 for 1 h. The blots were washed twice with TPBS and the signal detected typically between 1 to 15 min using chemiluminescence (Amersham, Buckinghamshire, UK).
Enzyme Assays
DHAR activity was assayed essentially as described (Hossain and Asada, 1984). Tobacco leaves were ground in extraction buffer (50 mm Tris-HCl, pH 7.4, 100 mm NaCl, 2 mm EDTA, 1 mm MgCl2) and soluble protein obtained following a 5-min centrifugation at 15,000g. DHAR was assayed from an equal amount of protein as described (Bradford, 1976) in 50 mm potassium phosphate, pH 6.5, 0.5 mm DHA, and 1 mm GSH, and its activity followed by an increase in A265. SOD (units to inhibit nitroblue tetrazolium photoreduction by 50%), CAT (10−6 mol H2O2 reduced min−1 mg−1 protein), and APX (10−8 mol Asc oxidized min−1 mg−1 protein) activities were determined as described (Gainnopolitis and Pies, 1977; Aebi, 1984; de Pinto et al., 2000). For the APX assay, 2 mm Asc was present in the extract buffer.
Asc and GSH Measurements
Asc was measured as described (Foyer et al., 1983). Apoplastic levels were obtained essentially as described (Pignocchi et al., 2003) in which leaves from 8-week-old tobacco were collected, the midvein removed, and 2-cm2 pieces vacuum infiltrated at −70 kPa with chilled 10 mm citrate, pH 3.0, for 10 min. Leaves were blotted dry, rolled, and centrifuged in a prechilled syringe at 500g for 10 min at 4°C. An equal volume of 2.5 m HClO4 (for the ascorbate assay) or 10% metaphoric acid (for the GSH assay) was added to the apoplastic wash fluid. For whole-leaf assays, leaves were ground in 2.5 m HClO4 and centrifuged at 15,000g for 10 min. Two volumes of 1.25 m Na2CO3 were added to the supernatant and, following centrifugation, 100 μL were added to 895 μL 100 mm potassium phosphate, pH 5.6. Asc was determined by the change in A265 following the addition of 0.25 unit ascorbate oxidase. The total amount of reduced and oxidized ascorbic acid (i.e. Asc and DHA) was determined by reducing DHA to Asc (in a reaction containing 100 mm potassium phosphate, pH 6.5, 2 mm GSH, and 0.1 μg recombinant wheat DHAR protein incubated at 25°C for 20 min) prior to measuring Asc. The amount of DHA was determined as the difference between these two assays. The level of GSH and GSSG was determined as described (Shimaoka et al., 2000).
Stomatal Measurements
Stomatal bioassay experiments were performed as described (Pei et al., 1997). Abaxial epidermis strips were loaded onto glass slides with 100 μL buffer A (50 mm KCl, 10 mm MES, pH 6.1). Following staining with 0.2% toluidine blue O for 20 s, the sample was rinsed twice with distilled water and imaged using a compound microscope (Leica, Wetzlar, Germany). For each sample, the percent of stomata that were open (defined as having a width greater than 1 μm) was determined from at least 400 stomata. The width and length of the opening (i.e. aperture) of only those stomata that were open were measured and used to calculate the average stomatal aperture (width/length). The width and length of at least 30 stomatal apertures were measured. The width and length of open stomatal apertures were also used to calculate the stomatal aperture area [π × (width/2) × (length/2)] which, together with the percentage of stomata that remained open, were used to calculate the stomatal area per unit leaf area containing 100 stomata [i.e. (average stomatal aperture area) × (percent of stomata that remained open) × 100].
H2O2 Measurements
Leaf discs (approximately 100 mg) were ground in liquid nitrogen, extracted in 150 μL of 25 mm HCl, and centrifuged at 15,000g for 5 min at 4°C. Pigments were removed by vortexing in the presence of activated charcoal and centrifugation at 12,000g for 15 min. H2O2 was determined by measuring relative fluorescence (excitation at 315 nm, emission at 425 nm) in a reaction containing 50 mm HEPES-NaOH, pH 7.5, 20 μL extract, 500 μm homovanillic acid, and 0.5 unit horseradish peroxidase against a standard curve as described (Creissen et al., 1999).
Leaf Measurements
In situ rates of CO2 assimilation, transpiration, and stomatal conductance were measured with a TPS-1 portable photosynthesis system (PP Systems, Amesbury, MA). Ambient growth conditions at the time of the measurements were 300 ppm CO2, 25°C, light intensity of 800 to 950 μmol photons m−2 s−1, and relative humidity of 60% to 70%.
Acknowledgments
We thank Dr. Tadahiko Mae for the gift of anti-Rubisco antiserum and Dr. Bruce Kohorn for the gift of anti-LHCP antiserum.
This work was supported by the National Research Initiative of the U.S. Department of Agriculture Cooperative State Research, Education and Extension Service (grant no. 2002–35100–12469) and the University of California Agricultural Experiment Station.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.062000.
References
- Aebi H (1984) Catalase in vitro. Methods Enzymol 105: 121–126 [DOI] [PubMed] [Google Scholar]
- Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50: 601–639 [DOI] [PubMed] [Google Scholar]
- Asada K (2000) The water-water cycle as alternative photon and electron sinks. Philos Trans R Soc Lond B Biol Sci 355: 1419–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl A, Kahl G (1995) Air pollutant stress changes the steady-state transcript levels of three photosynthesis genes. Environ Pollut 88: 57–65 [DOI] [PubMed] [Google Scholar]
- Ballach HJ, Oppenheimer S, Mooi J (1992) Reactions of cloned poplars to air pollution: premature leaf loss and investigations of the nitrogen metabolism. Z Naturforsch 47c: 109–119 [Google Scholar]
- Bowler C, Montague MV, Inze D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43: 83–116 [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Castillo FJ, Greppin H (1988) Extracellular ascorbic acid and enzyme activities related to ascorbic acid metabolism in Sedum album L. leaves after ozone exposure. Environ Exp Bot 28: 231–238 [Google Scholar]
- Chen Z, Gallie DR (2004) The ascorbic acid redox state controls guard cell signaling and stomatal movement. Plant Cell 16: 1143–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Young TE, Ling J, Chang S-C, Gallie DR (2003) Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc Natl Acad Sci USA 100: 3525–3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Last RL (1995) Differential accumulation of antioxidant mRNAs in Arabidopsis thaliana exposed to ozone. Plant Physiol 109: 203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Norris SR, Wheeler GL, Williams EH, Smirnoff N, Last RL (1999) Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis. Proc Natl Acad Sci USA 96: 4198–4203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Saracco SA, Norris SR, Last RL (2000) Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics 154: 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Williams EH, Last RL (1996) Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proc Natl Acad Sci USA 93: 9970–9974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoba-Pedregosa MC, Cordoba F, Villalba JM, Gonzales-Reyes JA (2003. a) Zonal changes in ascorbate and hydrogen peroxide contents, peroxidase, and ascorbate-related enzymes activities in onion roots. Plant Physiol 131: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoba-Pedregosa MC, Cordoba F, Villalba JM, Gonzales-Reyes JA (2003. b) Differential distribution of ascorbic acid, peroxidase activity, and hydrogen peroxide along the root axis in Allium cepa L. and its possible relationship with cell growth and differentiation. Protoplasma 221: 57–65 [DOI] [PubMed] [Google Scholar]
- Creissen G, Firmin J, Fryer M, Kular B, Leyland N, Reynolds H, Pastori G, Wellburn F, Baker N, Wellburn A, et al (1999) Elevated glutathione biosynthetic capacity in the chloroplasts of transgenic tobacco plants paradoxically causes increased oxidative stress. Plant Cell 11: 1277–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann MS, Pell EJ (1989) Decline of activity and quantity of ribulose bisphosphate carboxylase/oxygenase and net photosynthesis in ozone-treated potato foliage. Plant Physiol 91: 427–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pinto MC, De Gara L (2004) Changes in the ascorbate metabolism of apoplastic and symplastic spaces are associated with cell differentiation. J Exp Bot 55: 2559–2569 [DOI] [PubMed] [Google Scholar]
- de Pinto MC, Tommasi F, De Gara L (2000) Enzymes of the ascorbate biosynthesis and ascorbate-glutathione cycle in cultured cells of tobacco Bright Yellow 2. Plant Physiol Biochem 38: 541–550 [Google Scholar]
- Foyer CH, Rowell J, Walker D (1983) Measurements of the ascorbate content of spinach leaf protoplasts and chloroplasts during illumination. Planta 157: 239–244 [DOI] [PubMed] [Google Scholar]
- Freebairn HT (1960) The prevention of air pollution damage to plants by the use of vitamin C sprays. J Air Pollut Control Assoc 10: 314–317 [DOI] [PubMed] [Google Scholar]
- Freebairn HT, Taylor OC (1960) Prevention of plant damage from air-borne oxidizing agents. Proc Am Soc Hortic Sci 76: 693–699 [Google Scholar]
- Gainnopolitis CN, Pies SK (1977) Superoxide dismutases. I. Occurrence in higher plants. Plant Physiol 59: 309–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godde D, Buchhold J (1992) Effect of long-term fumigation with ozone on the turnover of the D1 reaction centre polypeptide of photosystem II in spruce (Picea abies). Physiol Plant 86: 568–574 [Google Scholar]
- Grabov A, Blatt MR (1998) Membrane voltage initiates Ca2+ waves and potentiates Ca2+ increases with abscisic acid in stomatal guard cells. Proc Natl Acad Sci USA 95: 4778–4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes HD, Perkins KK, Boss WF (1983) Ozone degrades into hydroxyl radical under physiological conditions. Plant Physiol 72: 1016–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan LM, Zhao J, Scandalios JG (2000) Cis-elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response. Plant J 22: 87–95 [DOI] [PubMed] [Google Scholar]
- Hamilton DW, Hills A, Kohler B, Blatt MR (2000) Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc Natl Acad Sci USA 97: 4967–4972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausladen A, Alscher RG (1993) Glutathione. In RG Alscher, J Hess, eds, Antioxidants in Higher Plants. CRC Press, Boca Raton, FL, pp 1–30
- Heagle AS (1989) Ozone and crop yield. Annu Rev Phytopathol 27: 397–423 [Google Scholar]
- Heath RL (1980) Initial events in injury to plants by air pollutants. Annu Rev Plant Physiol 31: 395–431 [Google Scholar]
- Heath RL (1987) The biochemistry of ozone attack on the plasma membranes of plant cells. Recent Adv Phytochem 21: 29–54 [Google Scholar]
- Heggestad HE, Middleton JT (1959) Ozone in high concentrations as cause of tobacco leaf injury. Science 129: 208–210 [DOI] [PubMed] [Google Scholar]
- Held AA, Mooney HA, Gorham JN (1991) Acclimation to ozone stress in radish: leaf demography and photosynthesis. New Phytol 118: 417–423 [Google Scholar]
- Herouart D, Bowler C, Tsang WTE, Van Camp W, Van Montagu M, Inzé D (1991) Differential expression of superoxide dismutase genes in Nicotiana plumbaginifolia exposed to environmental stress conditions. In E Pell, K Steffen, eds, Active Oxygen/Oxidative Stress and Plant Metabolism. American Society of Plant Physiologists, Rockville, MD, pp 250–252
- Hossain MA, Asada K (1984) Purification of dehydroascorbate reductase from spinach and its characterization as a thiol enzyme. Plant Cell Physiol 25: 85–92 [Google Scholar]
- Joo JH, Wang S, Chen JG, Jones AM, Fedoroff NV (2005) Different signaling and cell death roles of heterotrimeric G protein α and β subunits in the Arabidopsis oxidative stress response to ozone. Plant Cell 17: 957–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch JR, Creelman RA, Eshita SM, Seskar M, Mullet JE, Davis KR (2000) Ozone sensitivity in hybrid poplar correlates with insensitivity to both salicylic acid and jasmonic acid. The role of programmed cell death in lesion formation. Plant Physiol 123: 487–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler B, Blatt MR (2002) Protein phosphorylation activates the guard cell Ca2+ channel and is a prerequisite for gating by abscisic acid. Plant J 32: 185–194 [DOI] [PubMed] [Google Scholar]
- Kwon SY, Ahn YO, Lee HS, Kwak SS (2001) Biochemical characterization of transgenic tobacco plants expressing a human dehydroascorbate reductase gene. J Biochem Mol Biol 34: 316–321 [Google Scholar]
- Kwon SY, Choi SM, Ahn YO, Lee HS, Lee HB, Park YM, Kwak SS (2003) Enhanced stress-tolerance of transgenic tobacco plants expressing a human dehydroascorbate reductase gene. J Plant Physiol 160: 347–353 [DOI] [PubMed] [Google Scholar]
- Langebartels C, Kerner K, Leonardi S, Schraudner M, Trost M, Heller W, Sandermann H Jr (1990) Biochemical plant responses to ozone. I. Differential induction of polyamine and ethylene biosynthesis in tobacco. Plant Physiol 95: 882–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EH (1991) Plant resistance mechanisms to air pollutants: rhythms in ascorbic acid production during growth under ozone stress. Chronobiol Int 8: 93–102 [DOI] [PubMed] [Google Scholar]
- Lee EH, Jersey JA, Gifford C, Bennett J (1984) Differential ozone tolerance in soybean and snap beans: analysis of ascorbic acid in O3-susceptible and O3-resistant cultivars by high-performance liquid chromatography. Environ Exp Bot 24: 331–341 [Google Scholar]
- Loreto F, Mannozzi M, Maris C, Nascetti P, Ferranti F, Pasqualini S (2001) Ozone quenching properties of isoprene and its antioxidant role in leaves. Plant Physiol 126: 993–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luwe M, Heber U (1995) Ozone detoxification in the apoplasm and symplasm of spinach, bean and beech leaves at ambient and elevated concentrations of ozone in the air. Planta 197: 448–455 [Google Scholar]
- Luwe MWF, Takahama U, Heber U (1993) Role of ascorbate in detoxifying ozone in the apoplast of spinach (Spinacia oleracea L.) leaves. Plant Physiol 101: 969–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons T, Plochl M, Turcsanyi E, Barnes J (1999) Extracellular antioxidants: a protective screen against ozone? In S Agrawal, M Agrawal, eds, Environmental Pollution and Plant Responses. CRC Press/Lewis Publishers, Boca Raton, FL, pp 183–201
- Machler F, Wasescha MR, Krieg F, Oertli JJ (1995) Damage by ozone and protection by ascorbic acid in barley leaves. J Plant Physiol 147: 469–473 [Google Scholar]
- MacRobbie EA (2000) ABA activates multiple Ca(2+) fluxes in stomatal guard cells, triggering vacuolar K(+)(Rb(+)) release. Proc Natl Acad Sci USA 97: 12361–12368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh MR, Clayton H, Mansfield TA, Hetherington AM (1996) Changes in stomatal behavior and guard cell cytosolic free calcium in response to oxidative stress. Plant Physiol 111: 1031–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlhorn H, Tabner BJ, Wellburn AR (1990) Electron spin resonance: evidence for the formation of free radicals in plants exposed to ozone. Physiol Plant 79: 377–383 [Google Scholar]
- Menser HA (1964) Responses of plants to air pollutants. III. A relation between ascorbic acid levels and ozone susceptibility of light-preconditioned tobacco plants. Plant Physiol 39: 564–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake C, Asada K (1996) Inactivation mechanism of ascorbate peroxidase at low concentrations of ascorbate: hydrogen peroxide decomposes compound I of ascorbate peroxidase. Plant Cell Physiol 37: 423–430 [Google Scholar]
- Moldau H, Bichele I, Huve K (1998) Dark-induced ascorbate deficiency in leaf cell walls increases plasmalemma injury under ozone. Planta 207: 60–66 [Google Scholar]
- Moldau H, Padu E, Bichele I (1997) Quantification of ozone decay and requirement for ascorbate in Phaseolus vulgaris L. mesophyll cell walls. Phyton (Buenos Aires) 37: 175–180 [Google Scholar]
- Mudd JB (1997) Biochemical basis for the toxicity of ozone. In M Yunus, M Iqba, eds, Plant Response to Air Pollution. Wiley & Sons, New York, pp 267–284
- Muller-Moule P, Golan T, Niyogi KK (2004) Ascorbate-deficient mutants of Arabidopsis grow in high light despite chronic photooxidative stress. Plant Physiol 134: 1163–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Moule P, Havaux M, Niyogi KK (2003) Zeaxanthin deficiency enhances the high light sensitivity of an ascorbate-deficient mutant of Arabidopsis. Plant Physiol 133: 748–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Pei ZM, Mori IC, Schroeder J (2001) Abscisic acid activation of plasma membrane Ca(2+) channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 13: 2513–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie GY, Tomasevic M, Baker NR (1993) Effects of ozone on the photosynthetic apparatus and leaf proteins during leaf development in wheat. Plant Cell Environ 16: 643–651 [Google Scholar]
- Overmyer K, Tuominen H, Kettunen R, Betz C, Langebartels C, Sandermann H Jr, Kangasjarvi J (2000) Ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. Plant Cell 12: 1849–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualini S, Della Torre G, Ferranti F, Ederli L, Piccioni C, Reale L, Antonielli M (2002) Salicylic acid modulates ozone-induced hypersensitive cell death in tobacco plants. Physiol Plant 115: 204–212 [DOI] [PubMed] [Google Scholar]
- Pasqualini S, Piccioni C, Reale L, Ederli L, Della Torre G, Ferranti F (2003) Ozone-induced cell death in tobacco cultivar Bel W3 plants. The role of programmed cell death in lesion formation. Plant Physiol 133: 1122–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI (1997) Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell 9: 409–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Pell EJ, Enyedi A, Eckhardt N, Landry L (1990) Ozone induced alterations in quantity and activity of Rubisco: implications for foliar senescence. In CC Reddy, GA Hamilton, KM Madyastha, eds, Proceedings of International Symposium on Biological Oxidation Systems. Academic Press, San Diego, pp 389–403
- Pell EJ, Pearson NS (1983) Ozone-induced reduction in quantity of ribulose-1,5-bisphosphate carboxylase in alfalfa foliage. Plant Physiol 73: 185–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pell EJ, Schlagnhaufer CD, Arteca RN (1997) Ozone induced oxidative stress: mechanisms of action and reaction. Physiol Plant 100: 264–273 [Google Scholar]
- Pignocchi C, Fletcher JM, Wilkinson JE, Barnes JD, Foyer CH (2003) Function of ascorbate oxidase in tobacco. Plant Physiol 132: 1631–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino ME, Mudd JB, Bailey-Serres J (1995) Ozone-induced alterations in the accumulation of newly synthesized proteins in leaves of maize. Plant Physiol 108: 777–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polle A, Wieser G, Havranek WM (1995) Quantification of ozone influx and apoplastic ascorbate content in needles of Norway spruce trees (Picea abies L., Karst.) at high altitude. Plant Cell Environ 18: 681–688 [Google Scholar]
- Price AH, Taylor A, Ripley SJ, Griffiths A, Trewavas AJ, Knight MR (1994) Oxidative signals in tobacco increase cytosolic calcium. Plant Cell 6: 1301–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MV, Koch JR, Davis KR (2000) Ozone: a tool for probing programmed cell death in plants. Plant Mol Biol 44: 345–358 [DOI] [PubMed] [Google Scholar]
- Rao MV, Paliyath G, Ormrod DP (1995) Differential responses of photosynthetic pigments, Rubisco activity and Rubisco protein of Arabidopsis thaliana exposed to UV-B and ozone. Photochem Photobiol 62: 727–735 [Google Scholar]
- Reich PB (1983) Effects of low concentrations of O3 on net photosynthesis, dark respiration, and chlorophyll contents in aging hybrid poplar leaves. Plant Physiol 73: 291–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runeckles VC, Chevone BI (1992) Crop responses to ozone. In AS Lefohn, ed, Surface Level Ozone Exposures and Their Effects on Vegetation. Lewis Publishers, Chelsea, UK, pp 189–252
- Sakaki T, Kondo N, Sugahara K (1983) Breakdown of photosynthetic pigments and lipids in spinach leaves with ozone fumigation: role of active oxygens. Physiol Plant 59: 28–34 [Google Scholar]
- Sen Gupta A, Alscher RG, McCune D (1991) Response of photosynthesis and cellular antioxidants to ozone in Populus leaves. Plant Physiol 96: 650–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraudner M, Moeder W, Wiese C, Van Camp W, Inze D, Langebartels C, Sandermann H Jr (1998) Ozone-induced oxidative burst in the ozone biomonitor plant, tobacco Bel W3. Plant J 16: 235–245 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D (2001. a) Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 52: 627–658 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Kwak JM, Allen GJ (2001. b) Guard cell abscisic acid signaling and engineering drought hardiness in plants. Nature 410: 327–330 [DOI] [PubMed] [Google Scholar]
- Sharma YK, Davis KR (1994) Ozone-induced expression of stress-related genes in Arabidopsis thaliana. Plant Physiol 105: 1089–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimaoka T, Yokota A, Miyake C (2000) Purification and characterization of chloroplast dehydroascorbate reductase from spinach leaves. Plant Cell Physiol 41: 1110–1118 [DOI] [PubMed] [Google Scholar]
- Smirnoff N (2000) Ascorbic acid: metabolism and functions of a multi-faceted molecule. Curr Opin Plant Biol 3: 229–235 [PubMed] [Google Scholar]
- Tanaka K, Suda Y, Kondo N, Sugahara K (1985) O3 tolerance and the ascorbate-dependent H2O2 decomposing system in chloroplasts. Plant Cell Physiol 26: 1425–1431 [Google Scholar]
- Taylor GE Jr (1978) Plant and leaf resistance to gaseous air pollution stress. New Phytol 80: 523–534 [Google Scholar]
- Turcsanyi E, Lyons T, Plochl M, Barnes J (2000) Does ascorbate in the mesophyll cell walls form the first line of defence against ozone? Testing the concept using broad bean (Vicia faba L.). J Exp Bot 51: 901–910 [PubMed] [Google Scholar]
- Vanacker H, Carver TL, Foyer CH (1998) Pathogen-induced changes in antioxidant status of the apoplast in barley leaves. Plant Physiol 117: 1103–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veljovic-Jovanovic SD, Pignocchi C, Noctor G, Foyer CH (2001) Low ascorbic acid in the vtc-1 mutant of Arabidopsis is associated with decreased growth and intracellular redistribution of the antioxidant system. Plant Physiol 127: 426–435 [PMC free article] [PubMed] [Google Scholar]
- Willekens H, Van Camp W, Van Montagu M, Inze D, Langebartels C, Sandermann H Jr (1994) Ozone, sulfur dioxide, and ultraviolet B have similar effects on mRNA accumulation of antioxidant genes in Nicotiana plumbaginifolia L. Plant Physiol 106: 1007–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlgemuth H, Mittelstrass K, Kschieschan S, Bender J, Weigel H-J, Overmyer K, Kangasjärvi J, Sandermann H, Langebartels C (2002) Activation of an oxidative burst is a general feature of sensitive plants exposed to the air pollutant ozone. Plant Cell Environ 25: 717–726 [Google Scholar]
- Yamasaki H, Takahashi S, Heshiki R (1999) The tropical fig Ficus microcarpa L. f. cv. Golden Leaves lacks heat-stable dehydroascorbate reductase activity. Plant Cell Physiol 40: 640–646 [Google Scholar]
- Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song CP (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126: 1438–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]