Abstract
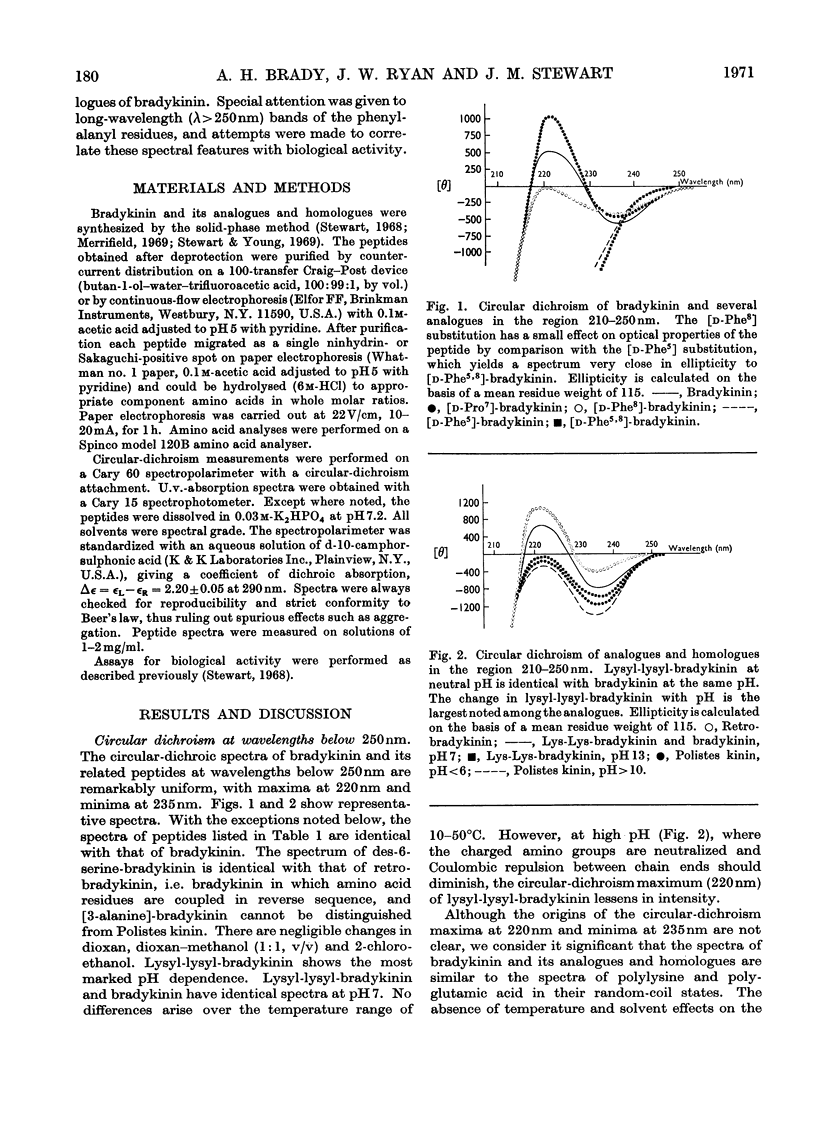
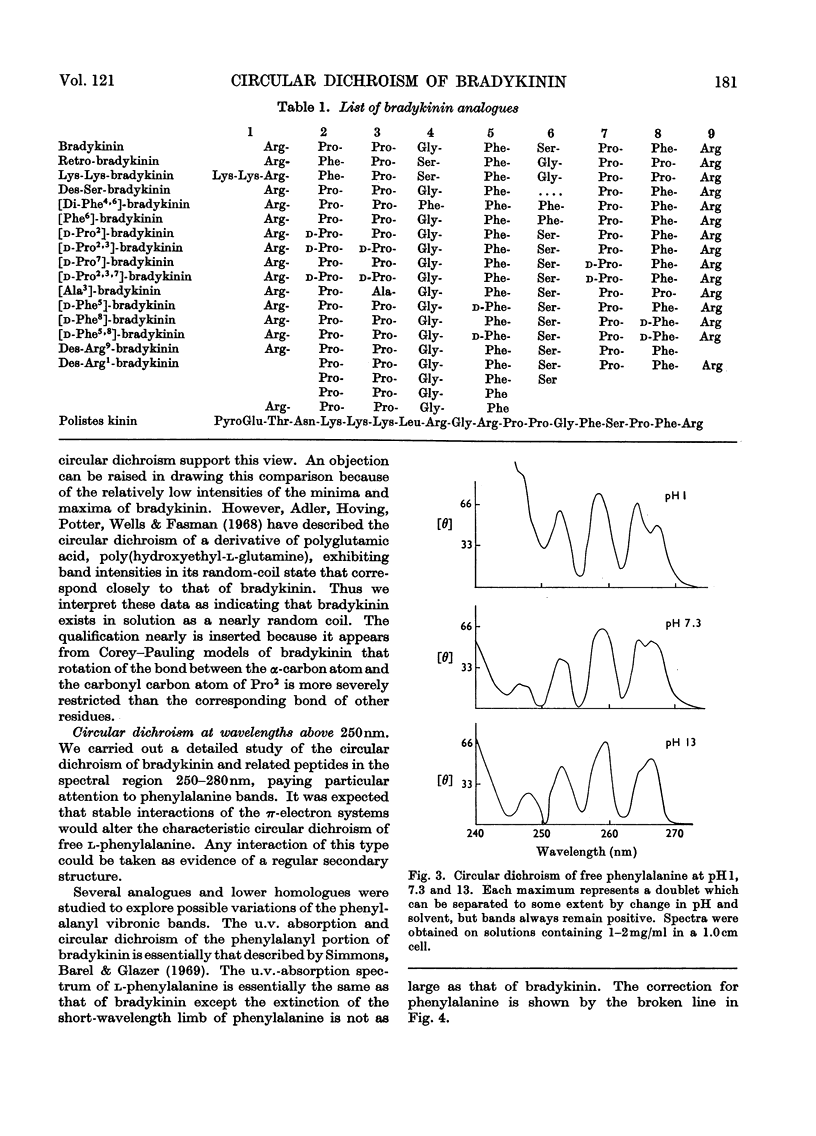
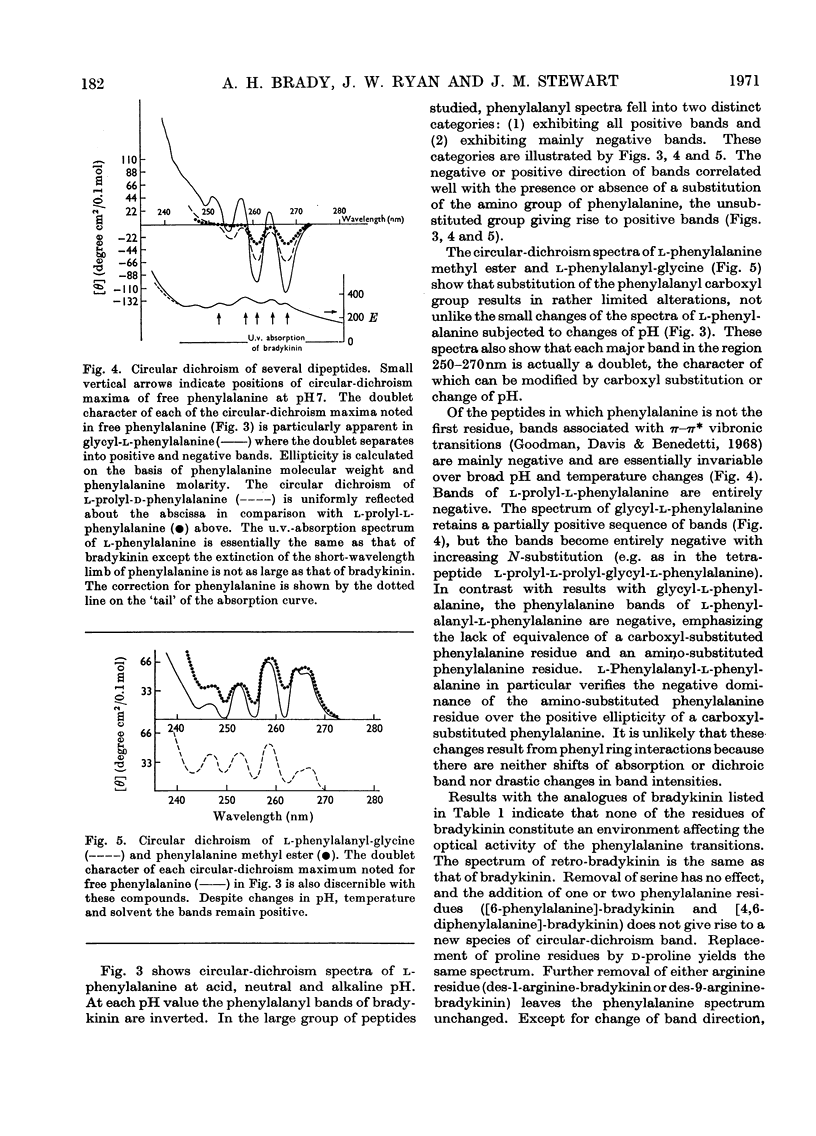
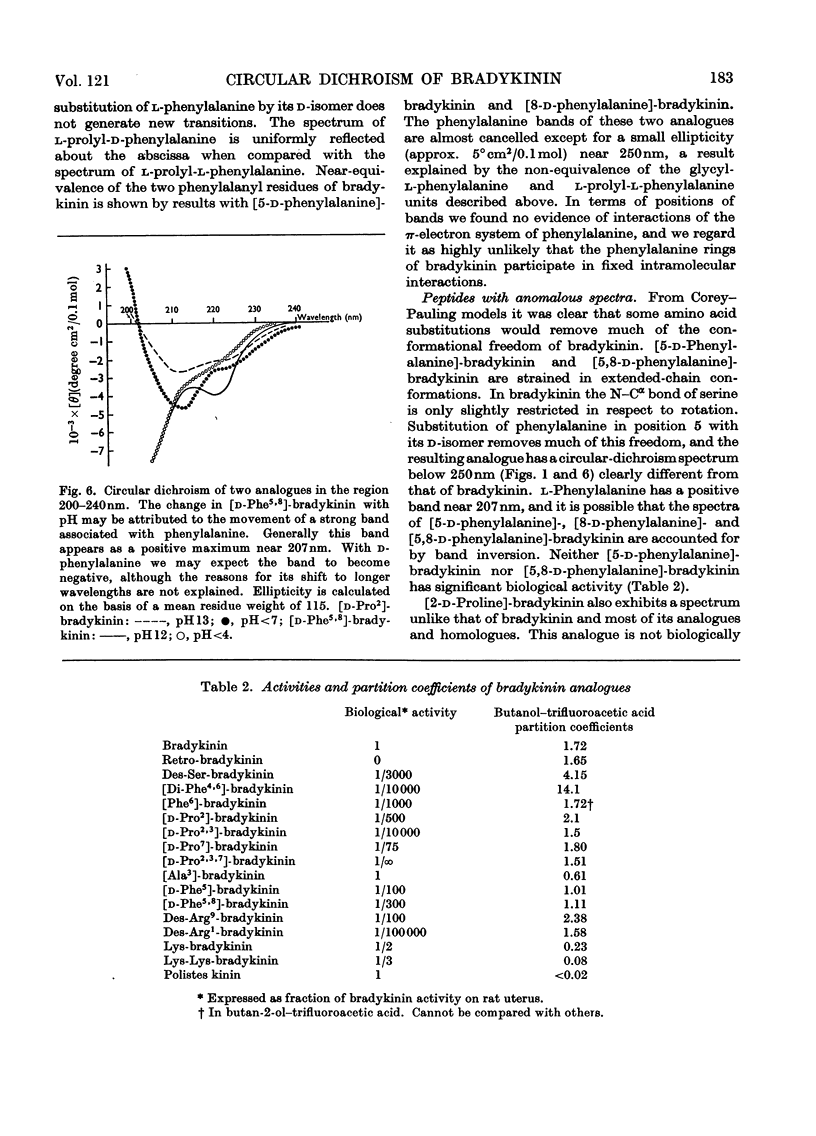
1. The circular dichroism of bradykinin and a number of its analogues and homologues was measured over the spectral range 200–300nm. All of the biologically active peptides showed maxima at 220nm and minima at 235nm. The spectra were independent of solvent and temperature. The vibronic transitions of phenylalanyl residues in the 250–280nm range showed no evidence of intra- or inter-molecular interactions. We take this as evidence that bradykinin and its biologically active analogues and homologues exist in solution as disordered chains. 2. None of the analogues with spectra unlike bradykinin possessed biological activity. However, peptides such as retro-bradykinin, des-6-serine-bradykinin, des-1-arginine-bradykinin and des-9-arginine-bradykinin produced spectra like that of bradykinin but were devoid of biological activity. Although we could not identify spectral features that were clearly correlated with biological activity, it appears unlikely that highly ordered peptides of the same amino acid composition as bradykinin would possess bradykinin-like effects.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler A. J., Hoving R., Potter J., Wells M., Fasman G. D. Circular dichroism of polypeptides. Poly(hydroxyethyl-L-glutamine) compared to poly(L-glutamic acid). J Am Chem Soc. 1968 Aug 14;90(17):4736–4738. doi: 10.1021/ja01019a045. [DOI] [PubMed] [Google Scholar]
- Merrifield R. B. Solid-phase peptide synthesis. Adv Enzymol Relat Areas Mol Biol. 1969;32:221–296. doi: 10.1002/9780470122778.ch6. [DOI] [PubMed] [Google Scholar]
- Simmons N. S., Barel A. O., Glazier A. N. High-resolution circular dichroism of N-acetyl-L-phenylalaninamide: solvent effects. Biopolymers. 1969;7(2):275–279. doi: 10.1002/bip.1969.360070213. [DOI] [PubMed] [Google Scholar]


