Abstract
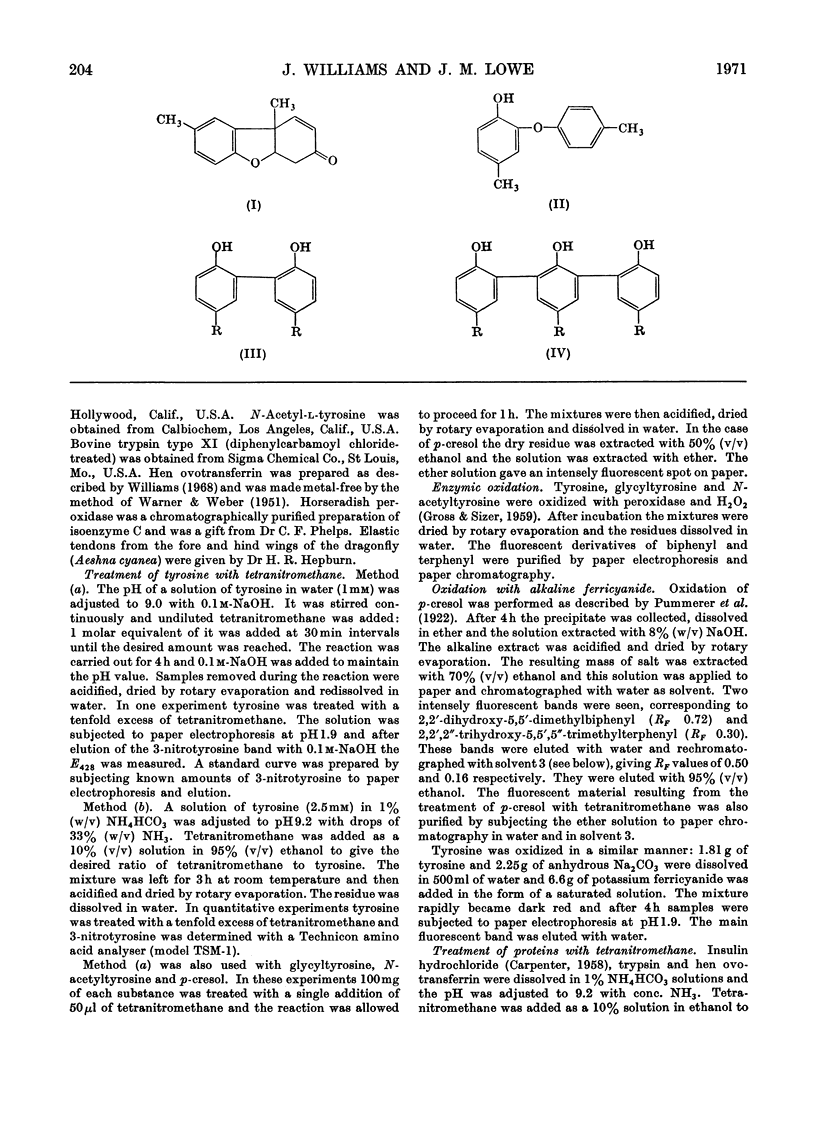
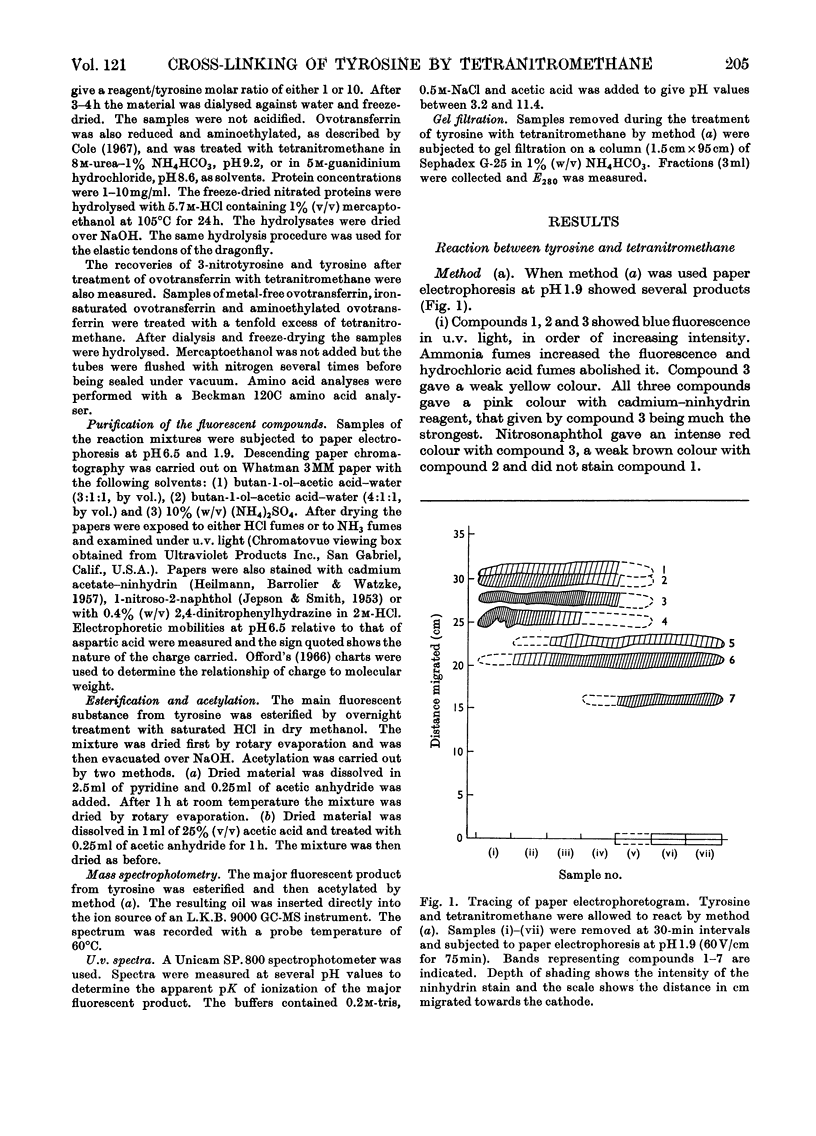
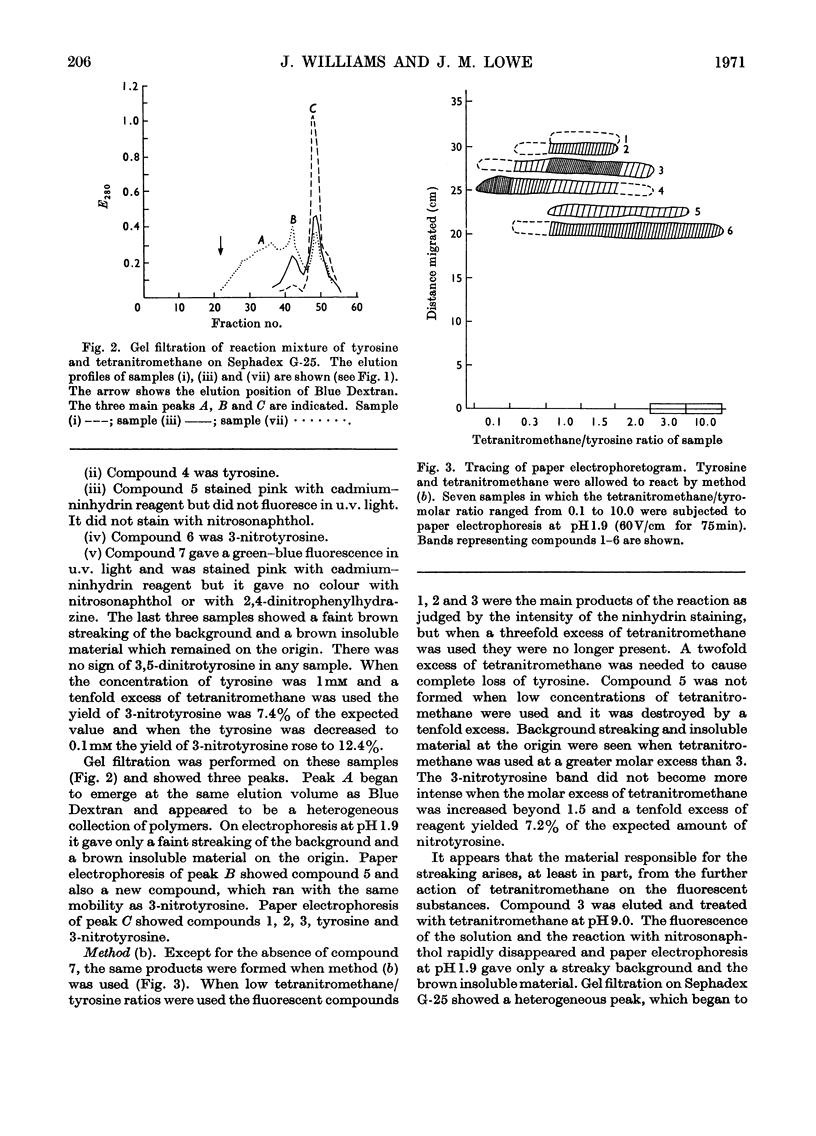
1. Tyrosine was treated with tetranitromethane. 2. Approx. 10% of the tyrosine was converted into 3-nitrotyrosine. 3. Three fluorescent compounds were also formed. They appear to be a dimer, trimer and tetramer in which tyrosine units are linked by biphenyl bonds. 4. The dimer and trimer have also been isolated from some proteins after treatment with tetranitromethane. 5. The yield of 3-nitrotyrosine from ovotransferrin after treatment with tetranitromethane was much smaller than the loss of tyrosine. 6. Several unidentified compounds were also formed by the reaction between tyrosine and tetranitromethane.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atassi M. Z., Habeeb A. F. Enzymic and immunochemical properties of lysozyme. I. Derivatives modified at tyrosine. Influence of nature of modification on activity. Biochemistry. 1969 Apr;8(4):1385–1393. doi: 10.1021/bi00832a012. [DOI] [PubMed] [Google Scholar]
- Boesel R. W., Carpenter F. H. Crosslinking during the nitration of bovine insulin with tetranitromethane. Biochem Biophys Res Commun. 1970 Feb 20;38(4):678–682. doi: 10.1016/0006-291x(70)90634-0. [DOI] [PubMed] [Google Scholar]
- Doyle R. J., Bello J., Roholt O. A. Probable protein crosslinking with tetranitromethane. Biochim Biophys Acta. 1968 Jun 26;160(2):274–276. doi: 10.1016/0005-2795(68)90103-7. [DOI] [PubMed] [Google Scholar]
- GROSS A. J., SIZER I. W. The oxidation of tyramine, tyrosine, and related compounds by peroxidase. J Biol Chem. 1959 Jun;234(6):1611–1614. [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- JEPSON J. B., SMITH I. Multiple dipping procedures in paper chromatography: a specific test for hydroxy-proline. Nature. 1953 Dec 12;172(4389):1100–1101. doi: 10.1038/1721100b0. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Sokolovsky M., Riordan J. F., Vallee B. L. Tetranitromethane. A reagent for the nitration of tyrosyl residues in proteins. Biochemistry. 1966 Nov;5(11):3582–3589. doi: 10.1021/bi00875a029. [DOI] [PubMed] [Google Scholar]
- Vincent J. P., Lazdunski M., Delaage M. On the use of tetranitromethane as a nitration reagent. The reaction of phenol side-chains in bovine and porcine trypsinogens and trypsins. Eur J Biochem. 1970 Feb;12(2):250–257. doi: 10.1111/j.1432-1033.1970.tb00844.x. [DOI] [PubMed] [Google Scholar]
- WARNER R. C., WEBER I. The preparation of crystalline conalbumin. J Biol Chem. 1951 Jul;191(1):173–180. [PubMed] [Google Scholar]
- Williams J. A comparison of glycopeptides from the ovotransferrin and serum transferrin of the hen. Biochem J. 1968 Jun;108(1):57–67. doi: 10.1042/bj1080057. [DOI] [PMC free article] [PubMed] [Google Scholar]


