Abstract
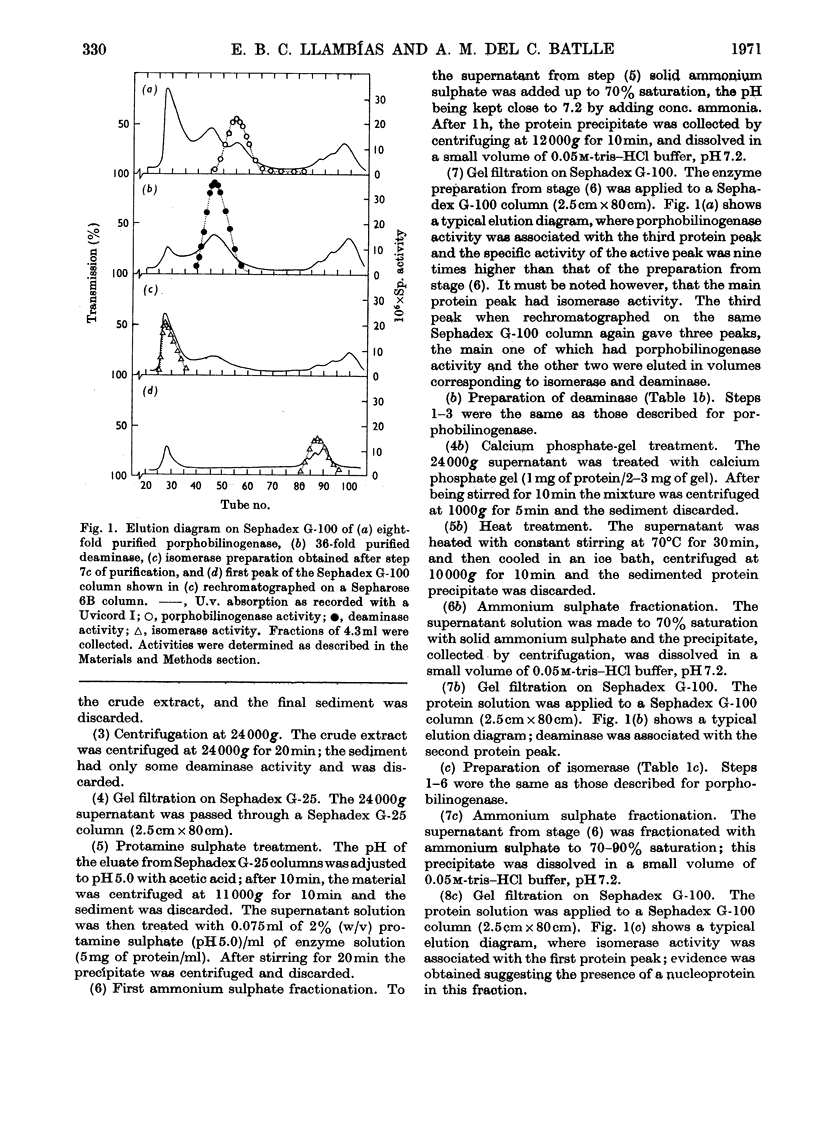
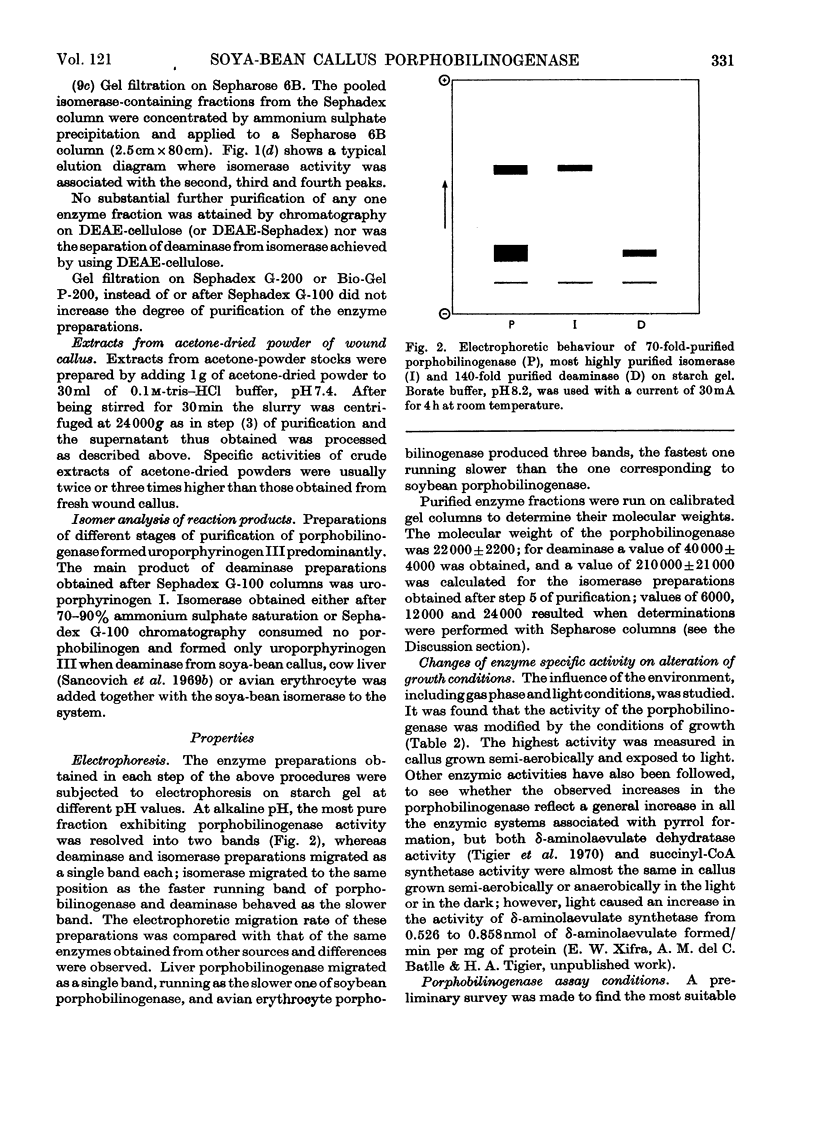
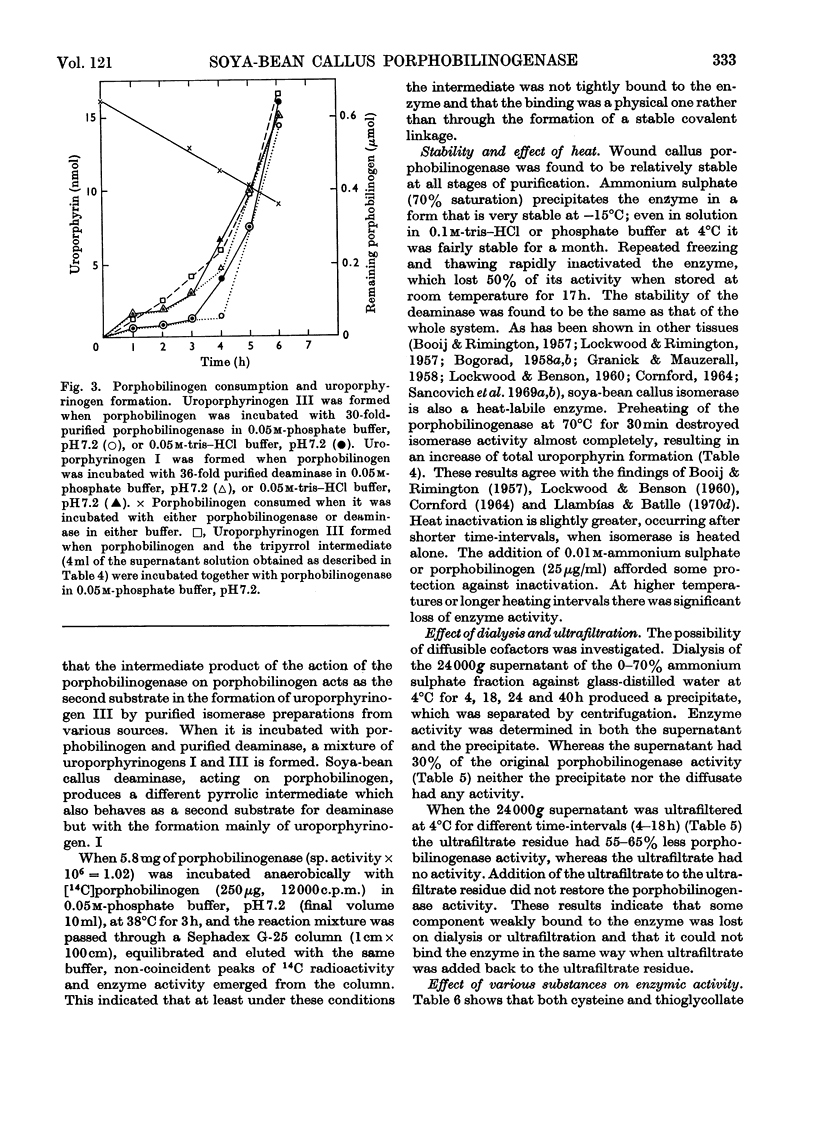
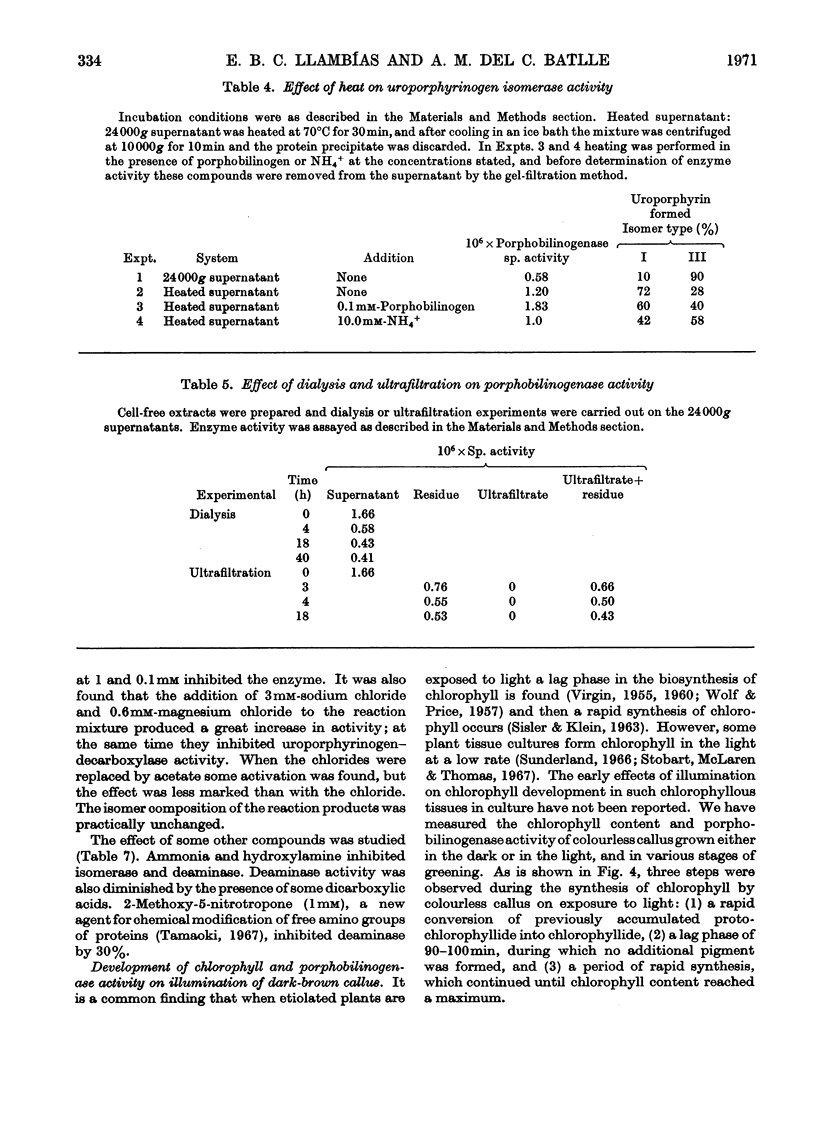
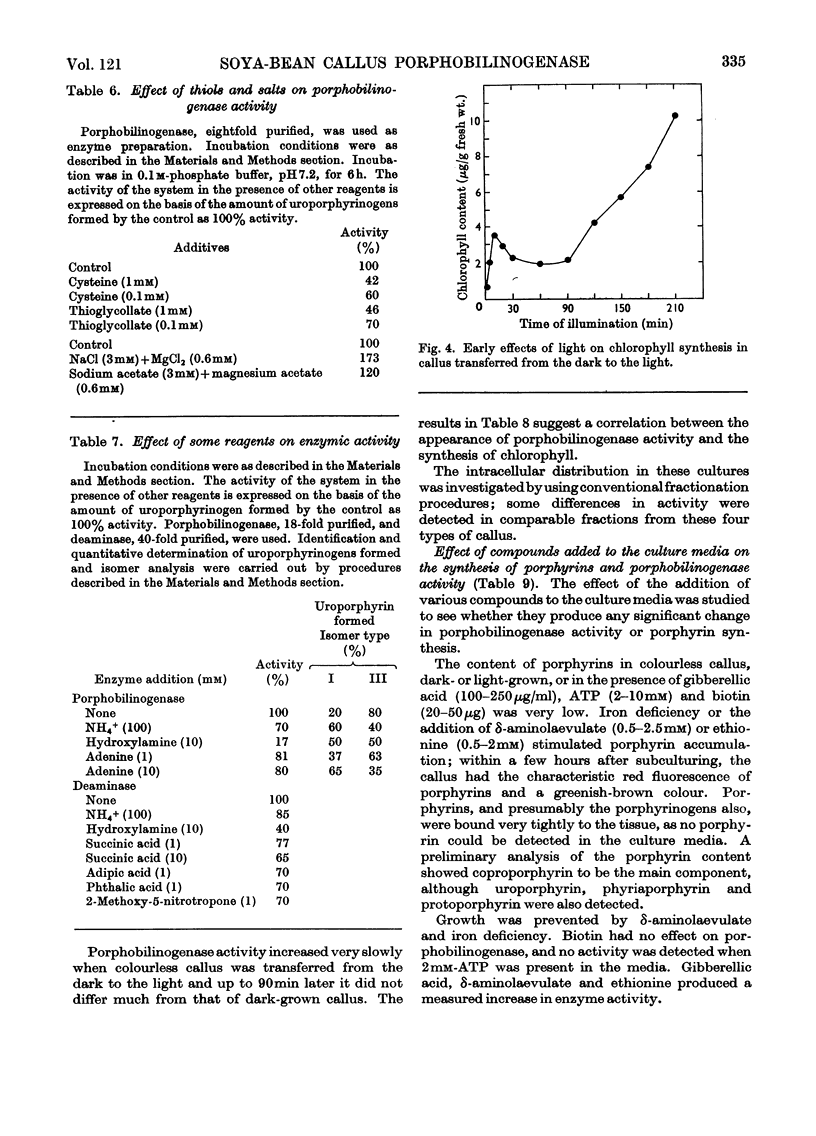
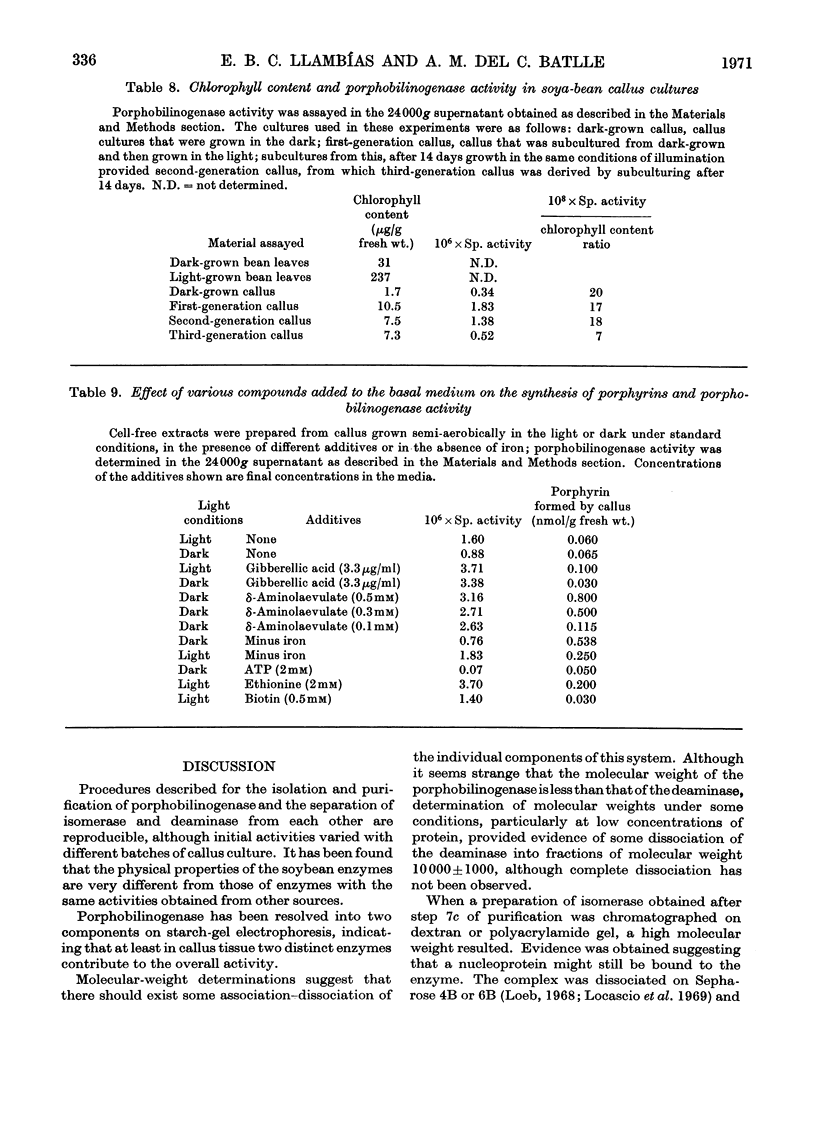
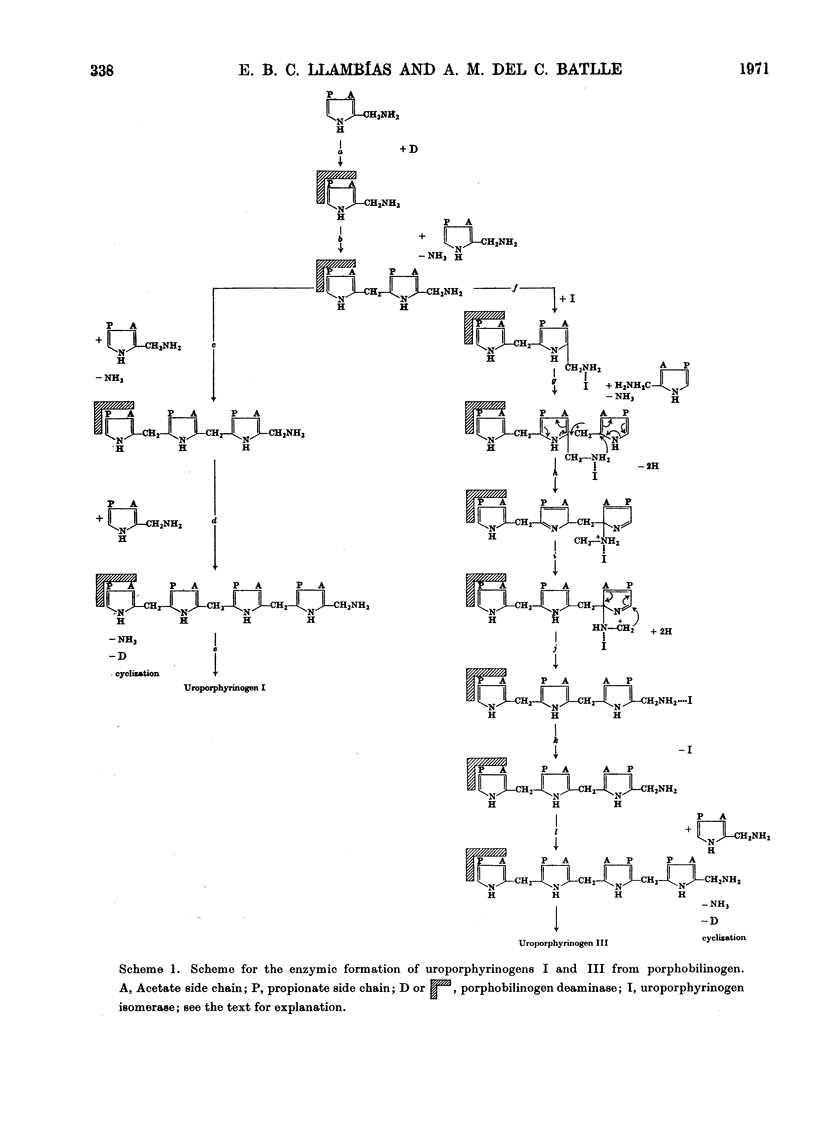
1. Porphobilinogenase was isolated and purified from soya-bean callus tissue; its components, porphobilinogen deaminase and uroporphyrinogen isomerase, were separated and purified. 2. The purified porphobilinogenase was resolved into two bands on starch-gel electrophoresis. The molecular weights of porphobilinogenase, deaminase and isomerase fractions were determined by the gel-filtration method. Porphobilinogenase activity was affected by the presence of air; uroporphyrinogens were only formed under anaerobic conditions, although substrate consumption was the same in the absence of oxygen as in its presence. 3. pH-dependence of both porphobilinogenase and deaminase was the same and a sharp optimum at pH 7.2 was obtained. Isomerase was heat-labile, but the presence of ammonium ions or porphobilinogen afforded some protection against inactivation. The action of several compounds added to the system was studied. Cysteine, thioglycollate, ammonium ions and hydroxylamine inhibited porphobilinogenase; certain concentrations of sodium and magnesium salts enhanced activity; some dicarboxylic acids and 2-methoxy-5-nitrotropone inhibited the deaminase. 4. δ-Aminolaevulate and ethionine in the culture media stimulated porphyrin synthesis and increased porphobilinogenase activity, whereas iron deficiency resulted in porphyrin accumulation. 5. The development of chlorophyll and porphobilinogenase on illumination of dark-grown callus was followed. 6. A hypothetical scheme is suggested for the enzymic synthesis of uroporphyrinogens from porphobilinogen.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOGORAD L., MARKS G. S. Studies on the biosynthesis of uroporphyrin III from porphobilinogen and the behaviour of uroporphyrin esters in paper chromatography. Biochim Biophys Acta. 1960 Jul 1;41:356–358. doi: 10.1016/0006-3002(60)90024-x. [DOI] [PubMed] [Google Scholar]
- BOGORAD L. The enzymatic synthesis of porphyrins from porphobilinogen. I. Uroporphyrin I. J Biol Chem. 1958 Aug;233(2):501–509. [PubMed] [Google Scholar]
- BOGORAD L. The enzymatic synthesis of porphyrins from porphobilinogen. II. Uroporphyrin III. J Biol Chem. 1958 Aug;233(2):510–515. [PubMed] [Google Scholar]
- Batlle A. M., Ferramola A. M., Grinstein M. Purification and general properties of sigma-aminolaevulate dehydratase from cow liver. Biochem J. 1967 Jul;104(1):244–249. doi: 10.1042/bj1040244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogorad L., Granick S. The Enzymatic Synthesis of Porphyrins from Porphobilinogen. Proc Natl Acad Sci U S A. 1953 Dec;39(12):1176–1188. doi: 10.1073/pnas.39.12.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOKSON G. H., RIMINGTON C. Porphobilinogen. Biochem J. 1954 Jul;57(3):476–484. doi: 10.1042/bj0570476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORNFORD P. A., BENSON A. A qualitative and quantitative study of the separation of uroporphyrin octamethyl esters I and III by dioxan chromatography. J Chromatogr. 1963 Feb;10:141–157. doi: 10.1016/s0021-9673(01)92287-3. [DOI] [PubMed] [Google Scholar]
- Cornford P. Transformation of porphobilinogen into porphyrins by preparations from human erythrocytes. Biochem J. 1964 Apr;91(1):64–73. doi: 10.1042/bj0910064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRESEL E. I., FALK J. E. Studies on the biosynthesis of blood pigments. 2. Haem and porphyrin formation in intact chicken erythrocytes. Biochem J. 1956 May;63(1):72–79. doi: 10.1042/bj0630072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Batlle A. M., Benson A. A study of the separation of uroporphyrin octamethyl esters I and 3 by thin-layer and paper chromatography. J Chromatogr. 1966 Nov;25(1):117–123. doi: 10.1016/s0021-9673(01)98224-x. [DOI] [PubMed] [Google Scholar]
- FALK J. E., DRESEL E. I., RIMINGTON C. Porphobilinogen as a porphyrin precursor, and interconversion of porphyrins, in a tissue system. Nature. 1953 Aug 15;172(4372):292–294. doi: 10.1038/172292a0. [DOI] [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., TAIT G. H. Studies on the biosynthesis of porphyrin and bacteriochlorophyll by Rhodopseudomonas spheroides. 1. The effect of growth conditions. Biochem J. 1962 Jun;83:539–549. doi: 10.1042/bj0830539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., TAIT G. H. Studies on the biosynthesis of porphyrin and bacteriochlorophyll by Rhodopseudomonas spheroides. 2. The effects of ethionine and threonine. Biochem J. 1962 Jun;83:550–559. doi: 10.1042/bj0830550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANICK S. Enzymatic conversion of delta-amino levulinic acid to porphobilinogen. Science. 1954 Dec 31;120(3131):1105–1106. doi: 10.1126/science.120.3131.1105. [DOI] [PubMed] [Google Scholar]
- GRANICK S., MAUZERALL D. Pbrphyrin biosynthesis in erythrocytes. II. Enzymes converting gamma-aminolevulinic acid to coproporphyrinogen. J Biol Chem. 1958 Jun;232(2):1119–1140. [PubMed] [Google Scholar]
- Gajdos A., Gajdos-Török M., Gorchein A., Neuberger A., Tait G. H. The effect of adenosine triphosphate on porphyrin excretion and on glycine metabolism in Rhodopseudomonas spheroides. Biochem J. 1968 Jan;106(1):185–192. doi: 10.1042/bj1060185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassman M., Bogorad L. Control of chlorophyll production in rapidly greening bean leaves. Plant Physiol. 1967 Jun;42(6):774–780. doi: 10.1104/pp.42.6.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEATH H., HOARE D. S. The biosynthesis of porphyrins from porphobilinogen by Rhodopseudomonas spheroides. Biochem J. 1959 May;72(1):14–22. doi: 10.1042/bj0720014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOARE D. S., HEATH H. The biosynthesis of porphyrins from porphobilinogen by Rhodopseudomonas spheroides. Biochem J. 1959 Dec;73:679–690. doi: 10.1042/bj0730679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEILIN D., HARTREE E. F. Purification of horse-radish peroxidase and comparison of its properties with those of catalase and methaemoglobin. Biochem J. 1951 Jun;49(1):88–104. doi: 10.1042/bj0490088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASCELLES J. Adaptation to form bacteriochlorophyll in Rhodopseudomonas spheroides: changes in activity of enzymes concerned in pyrrole synthesis. Biochem J. 1959 Jul;72:508–518. doi: 10.1042/bj0720508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOCKWOOD W. H., BENSON A. The enzymic condensation of porphobilinogen to porphyrins. Biochem J. 1960 May;75:372–381. doi: 10.1042/bj0750372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lascelles J., Hatch T. P. Bacteriochlorophyll and heme synthesis in Rhodopseudomonas spheroides: possible role of heme in regulation of the branched biosynthetic pathway. J Bacteriol. 1969 May;98(2):712–720. doi: 10.1128/jb.98.2.712-720.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E. Y. Uroporphyrinogen 3 cosynthetase from mouse spleen. Biochemistry. 1968 Nov;7(11):3781–3788. doi: 10.1021/bi00851a001. [DOI] [PubMed] [Google Scholar]
- Llambias E. B.C., del C Batlle A. M. Uroporphyrinogen III cosynthetase. Evidence for the existence of a polypyrrolic substrate in soybean callus tissue. FEBS Lett. 1970 Feb 16;6(3):285–288. doi: 10.1016/0014-5793(70)80079-5. [DOI] [PubMed] [Google Scholar]
- Llambías E. B.C., del C Batlle A. M. Negative homotropic kinetics of soybean callus porphobilinogen deaminase-uroporphyrinogen III cosynthetase. FEBS Lett. 1970 Sep 6;9(3):180–182. doi: 10.1016/0014-5793(70)80349-0. [DOI] [PubMed] [Google Scholar]
- Locascio G. A., Tigier H. A., Battle AM del C. Estimation of molecular weights of proteins by agarose gel filtration. J Chromatogr. 1969 Apr 8;40(3):453–457. doi: 10.1016/s0021-9673(01)96685-3. [DOI] [PubMed] [Google Scholar]
- Loeb J. E. Gel filtration of deoxyribonucleoprotein on agarose. Biochim Biophys Acta. 1968 Apr 22;157(2):424–426. doi: 10.1016/0005-2787(68)90099-3. [DOI] [PubMed] [Google Scholar]
- MOORE D. J., LABBE R. F. A QUANTITATIVE ASSAY FOR URINARY PORPHOBILINOGEN. Clin Chem. 1964 Dec;10:1105–1111. [PubMed] [Google Scholar]
- Rimington C., Benson A. Partition of porphyrins between cyclohexanone and aqueous sodium acetate as a function of pH. Determination of uroporphyrin and of hydrophilic porphyrin conjugates. Biochem J. 1967 Dec;105(3):1085–1090. doi: 10.1042/bj1051085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimington C. Spectral-absorption coefficients of some porphyrins in the Soret-band region. Biochem J. 1960 Jun;75(3):620–623. doi: 10.1042/bj0750620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancovich H. A., Battle A. M., Grinstein M. Porphyrin biosynthesis. VI. Separation and purification of porphobilinogen deaminase and uroporphyrinogen isomerase from cow liver. Porphobilinogenase an allosteric enzyme. Biochim Biophys Acta. 1969 Sep 30;191(1):130–143. doi: 10.1016/0005-2744(69)90322-2. [DOI] [PubMed] [Google Scholar]
- Stevens E., Frydman R. B., Frydman B. Separation of porphobilinogen deaminase and uroporphyrinogen 3 cosynthetase from human erythrocytes. Biochim Biophys Acta. 1968 Jun 24;158(3):496–498. doi: 10.1016/0304-4165(68)90314-0. [DOI] [PubMed] [Google Scholar]
- Tigier H. A., Batlle A. M., Locascio G. A. Porphyrin biosynthesis in the soybean callus tissue system. II. Improved purification and some properties of delta amino-laevulic acid dehydratase. Enzymologia. 1970 Jan 30;38(1):43–56. [PubMed] [Google Scholar]
- Tigier H. A., Del Batlle A. M., Locascio G. Porphyrin biosynthesis in soybean callus tissue system. Isolation, purification and general properties of delta-aminolaevulinate dehydratase. Biochim Biophys Acta. 1968 Jan 8;151(1):300–302. doi: 10.1016/0005-2744(68)90193-9. [DOI] [PubMed] [Google Scholar]
- WILDY J., NIZET A., BENSON A. Identification of a plasma material stimulating haemoglobin synthesis in vitro. Biochim Biophys Acta. 1961 Dec 23;54:414–423. doi: 10.1016/0006-3002(61)90080-4. [DOI] [PubMed] [Google Scholar]
- WOLFF J. B., PRICE L. Terminal steps of chlorophyll A biosynthesis in higher plants. Arch Biochem Biophys. 1957 Dec;72(2):293–301. doi: 10.1016/0003-9861(57)90205-9. [DOI] [PubMed] [Google Scholar]
- Wider E. A., Tigier H. A. Properties and regulatory effect on tetrapyrrole biosynthesis of succinyl CoA synthetase isolated from soybean callus tissue system. FEBS Lett. 1970 Jul 15;9(1):30–32. doi: 10.1016/0014-5793(70)80303-9. [DOI] [PubMed] [Google Scholar]
- del Batlle A. M., Benson A., Rimington C. Purification and properties of coproporphyrinogenase. Biochem J. 1965 Dec;97(3):731–740. doi: 10.1042/bj0970731. [DOI] [PMC free article] [PubMed] [Google Scholar]


