Abstract
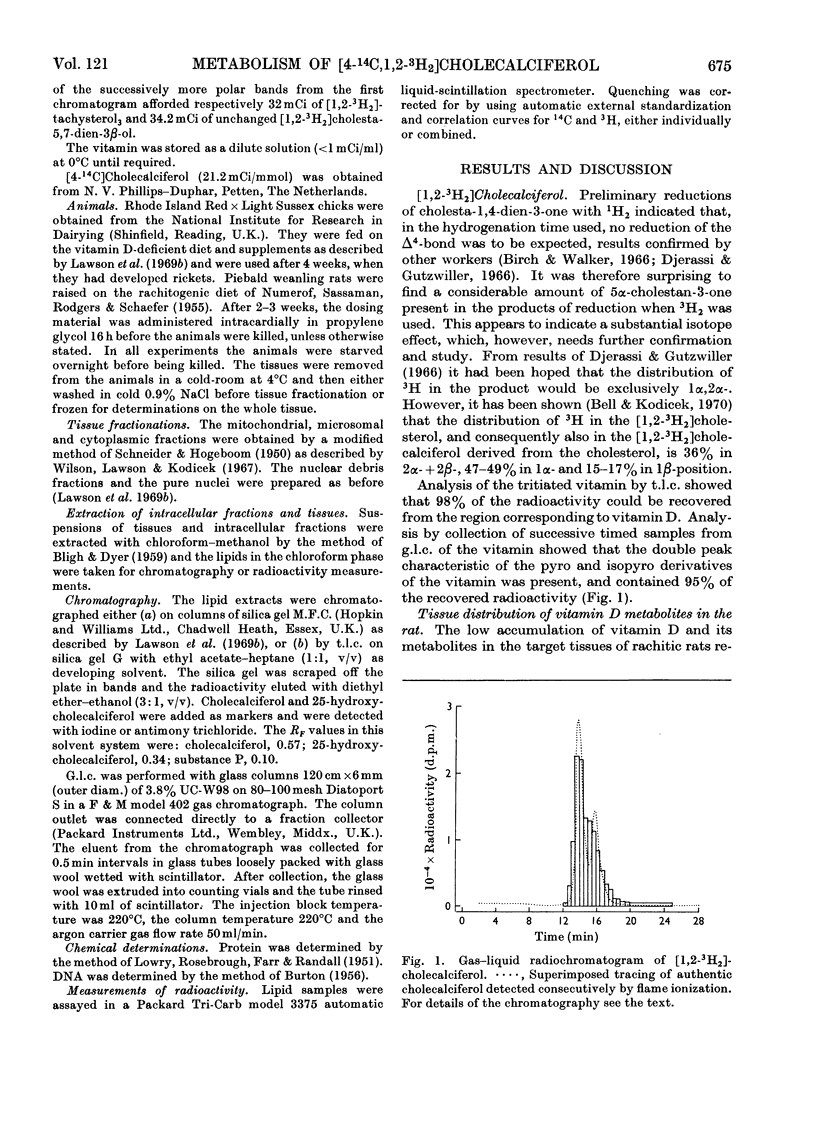
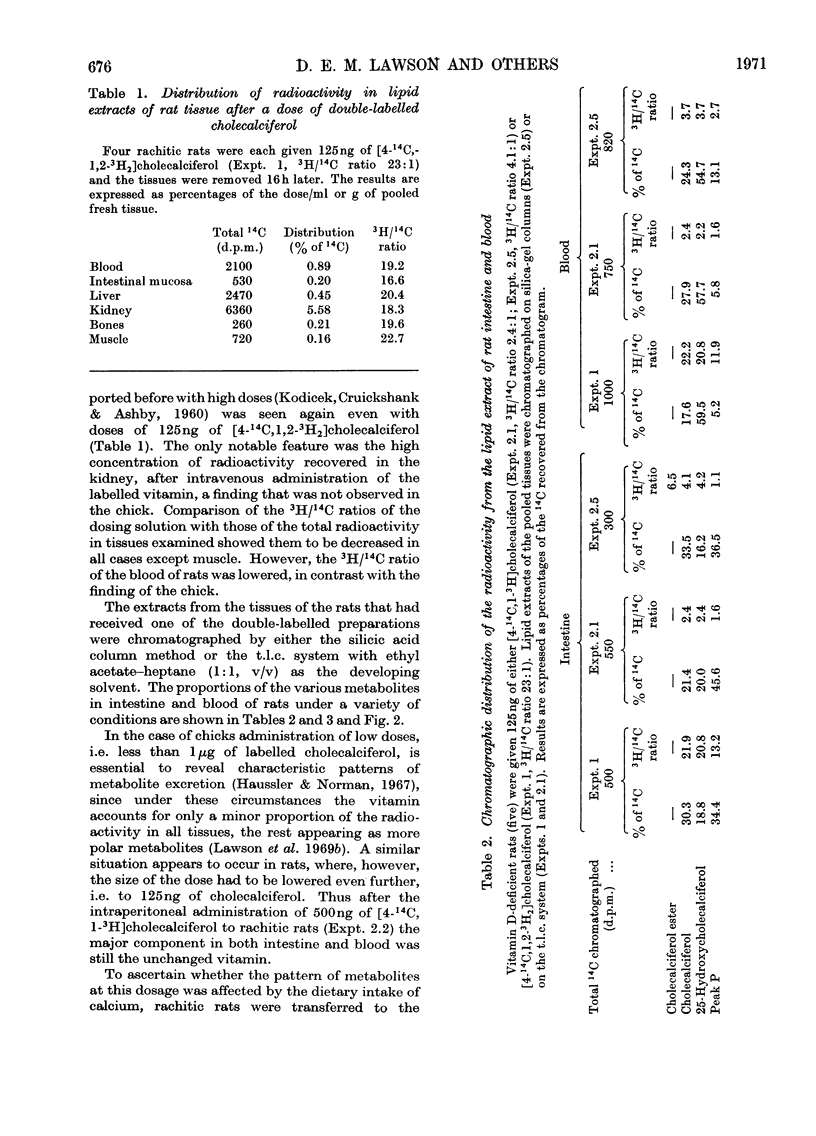
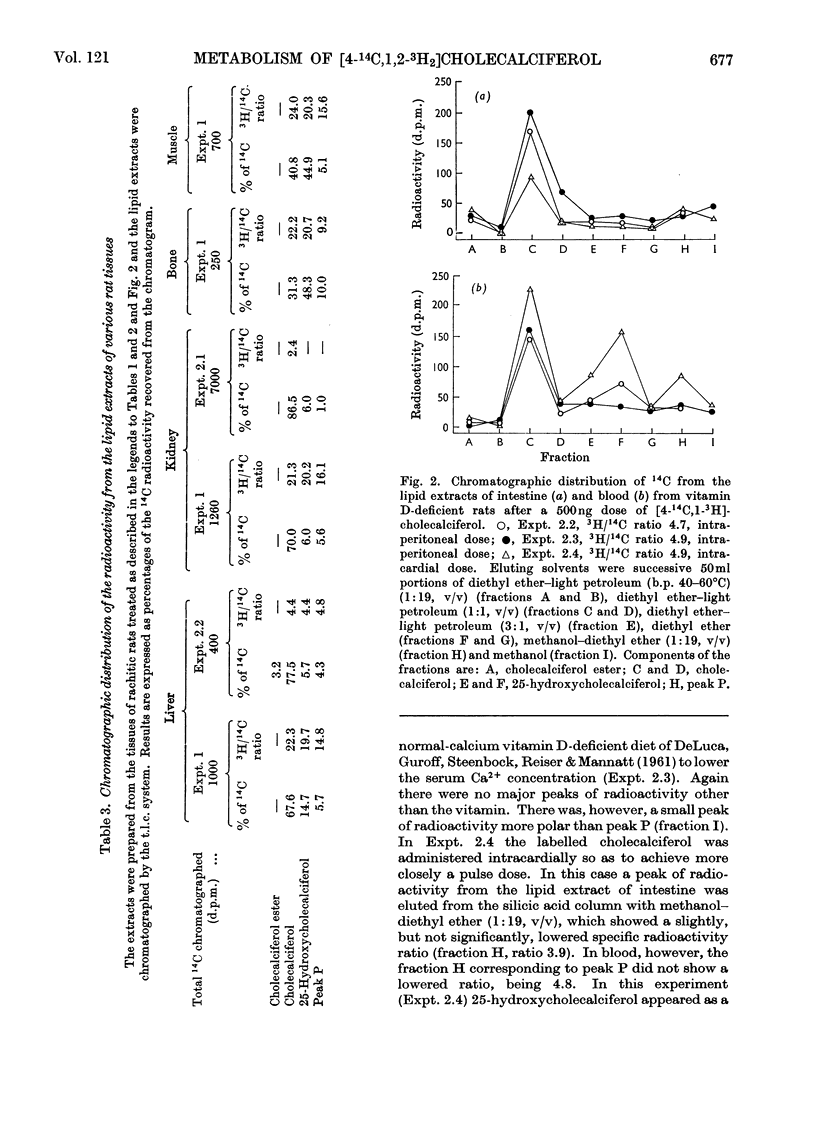
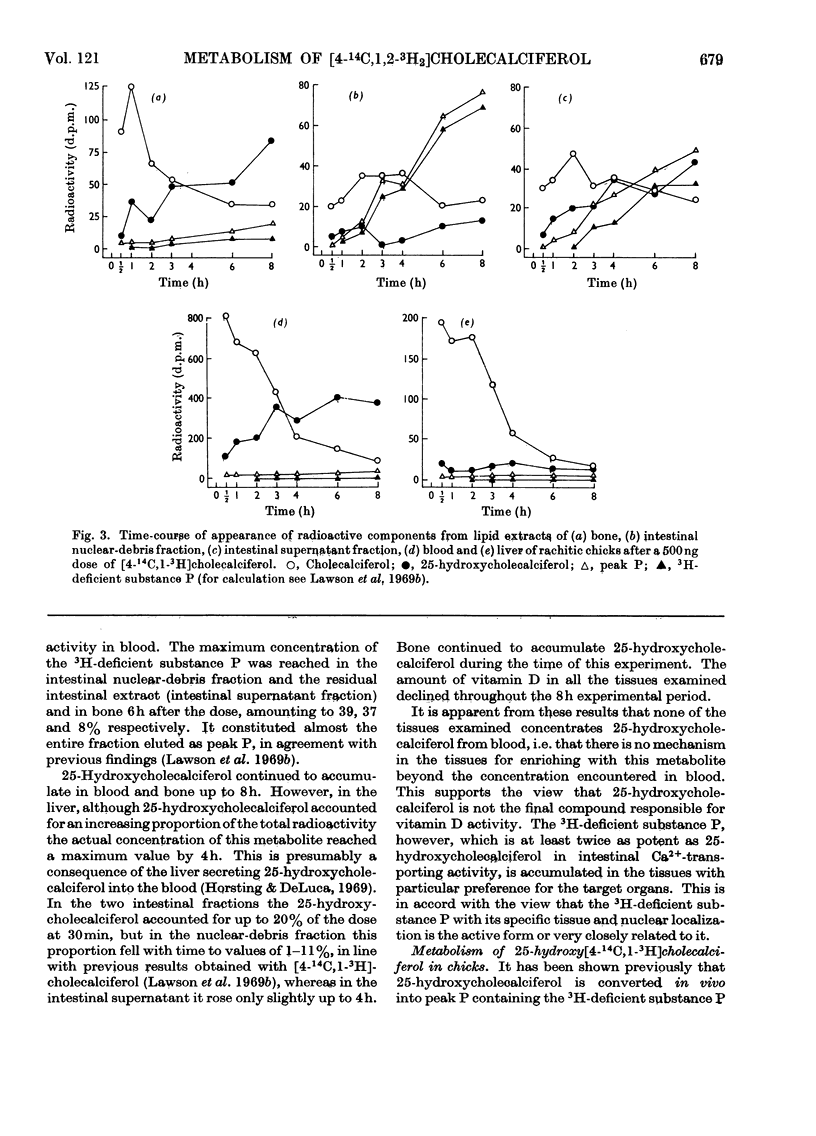
[1,2-3H2]Cholecalciferol has been synthesized with a specific radioactivity of 508mCi/mmol by using tristriphenylphosphinerhodium chloride, the homogeneous hydrogen catalyst. With doses of 125ng (5i.u.) of [4-14C,1-3H2]cholecalciferol the tissue distribution in rachitic rats of cholecalciferol and its metabolites (25-hydroxycholecalciferol and peak P material) was similar to that found in chicken with 500ng doses of the double-labelled vitamin. The only exceptions were rat kidney, with a very high concentration of vitamin D, and rat blood, with a higher proportion of peak P material, containing a substance formed from vitamin D with the loss of hydrogen from C-1. Substance P formed from [4-14C,1,2-3H2]cholecalciferol retained 36% of 3H, the amount expected from its distribution between C-1 and C-2, the 3H at C-1 being lost. 25-Hydroxycholecalciferol does not seem to have any specific intracellular localization within the intestine of rachitic chicks. The 3H-deficient substance P was present in the intestine and bone 1h after a dose of vitamin D and 30min after 25-hydroxycholecalciferol. There was very little 25-hydroxycholecalciferol in intestine at any time-interval, but bone and blood continued to take it up over the 8h experimental period. It is suggested that the intestinal 3H-deficient substance P originates from outside this tissue. The polar metabolite found in blood and which has retained its 3H at C-1 is not a precursor of the intestinal 3H-deficient substance P.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell P. A., Kodicek E. Investigations on metabolites of vitamin D in rat bile. Separation and partial identification of a major metabolite. Biochem J. 1969 Dec;115(4):663–669. doi: 10.1042/bj1150663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell P. A., Kodicek E. The stereospecificity of tritium distribution in [1-3H]- and [1,2-3H2]-cholesterol and -cholecalciferol. Biochem J. 1970 Feb;116(4):755–757. doi: 10.1042/bj1160755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins R. J., DeLuca H. F., Chen T., Suda T., Tanaka Y. Metabolism and subcellular location of 25-hydroxycholecalciferol in intestinal mucosa. Biochemistry. 1970 Mar 17;9(6):1453–1459. doi: 10.1021/bi00808a021. [DOI] [PubMed] [Google Scholar]
- DE LUCA H. F., GUROFF G., STEENBOCK H., REISER S., MANNATT M. R. Effect of various vitamin deficiencies on citric acid metabolism in the rat. J Nutr. 1961 Oct;75:175–180. doi: 10.1093/jn/75.2.175. [DOI] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Enzyme studies on the esterification of vitamin D in rat tissues. Biochem J. 1968 Sep;109(3):457–467. doi: 10.1042/bj1090457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Investigations on vitamin D esters synthesized in rats. Turnover and sites of synthesis. Biochem J. 1968 Jan;106(2):491–496. doi: 10.1042/bj1060491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Investigations on vitamin D esters synthesized rats. Detection and identification. Biochem J. 1968 Jan;106(2):485–490. doi: 10.1042/bj1060485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970 Nov 21;228(5273):764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- Horsting M., DeLuca H. F. In vitro production of 25-hydroxycholecalciferol. Biochem Biophys Res Commun. 1969 Jul 23;36(2):251–256. doi: 10.1016/0006-291x(69)90322-2. [DOI] [PubMed] [Google Scholar]
- Kodicek E., Lawson D. E., Wilson P. W. Biological activity of a polar metabolite of vitamin D. Nature. 1970 Nov 21;228(5273):763–764. doi: 10.1038/228763a0. [DOI] [PubMed] [Google Scholar]
- Lawson D. E., Wilson P. W., Kodicek E. Metabolism of vitamin D. A new cholecalciferol metabolite, involving loss of hydrogen at C-1, in chick intestinal nuclei. Biochem J. 1969 Nov;115(2):269–277. doi: 10.1042/bj1150269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson D. E., Wilson P. W., Kodicek E. New vitamin D metabolite localized in intestinal cell nuclei. Nature. 1969 Apr 12;222(5189):171–172. doi: 10.1038/222171a0. [DOI] [PubMed] [Google Scholar]
- Myrtle J. F., Haussler M. R., Norman A. W. Evidence for the biologically active form of cholecalciferol in the intestine. J Biol Chem. 1970 Mar 10;245(5):1190–1196. [PubMed] [Google Scholar]
- NUMEROF P., SASSAMAN H. L., RODGERS A., SCHAEFER A. E. The use of radioactive phosphorus in the assay of vitamin D. J Nutr. 1955 Jan;55(1):13–21. doi: 10.1093/jn/55.1.13. [DOI] [PubMed] [Google Scholar]
- Neville P. F., DeLuca H. F. The synthesis of [1,2-3H]vitamin D3 and the tissue localization of a 0.25-mu-g (10 IU) dose per rat. Biochemistry. 1966 Jul;5(7):2201–2207. doi: 10.1021/bi00871a007. [DOI] [PubMed] [Google Scholar]
- Suda T., DeLuca H. F., Schnoes H. K., Ponchon G., Tanaka Y., Holick M. F. 21,25-dihydroxycholecalciferol. A metabolite of vitamin D3 preferentially active on bone. Biochemistry. 1970 Jul 7;9(14):2917–2922. doi: 10.1021/bi00816a025. [DOI] [PubMed] [Google Scholar]
- Wilson P. W., Lawson D. E., Kodicek E. The intracellular distribution of [1-3H]cholecalciferol in the intestine of vitamin D-deficient and -supplemented rats. Biochem J. 1967 Apr;103(1):165–171. doi: 10.1042/bj1030165. [DOI] [PMC free article] [PubMed] [Google Scholar]


