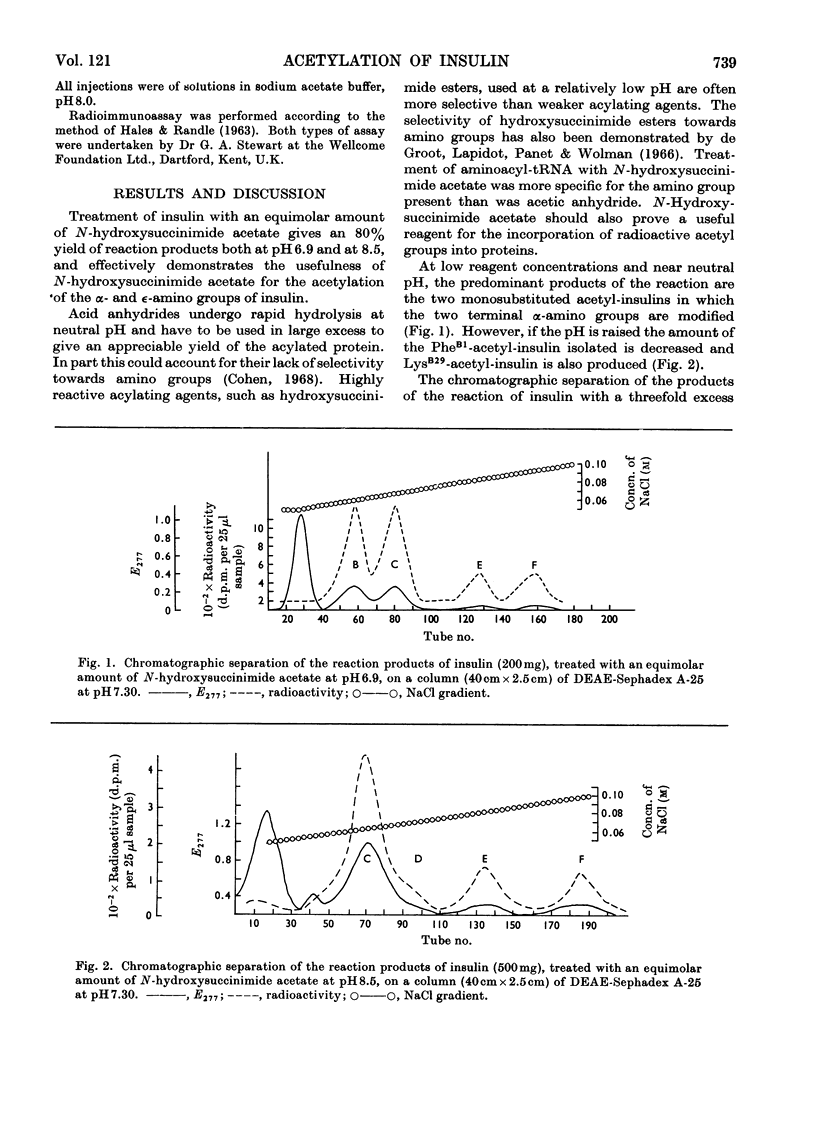
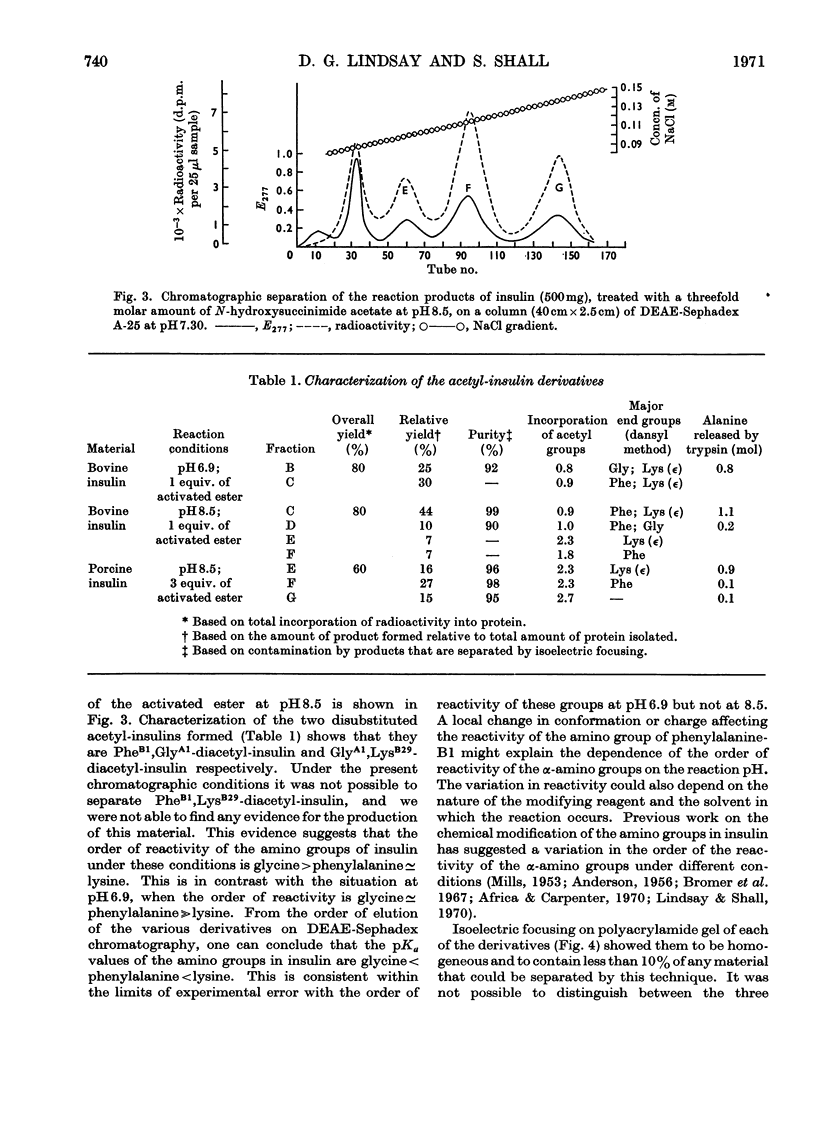
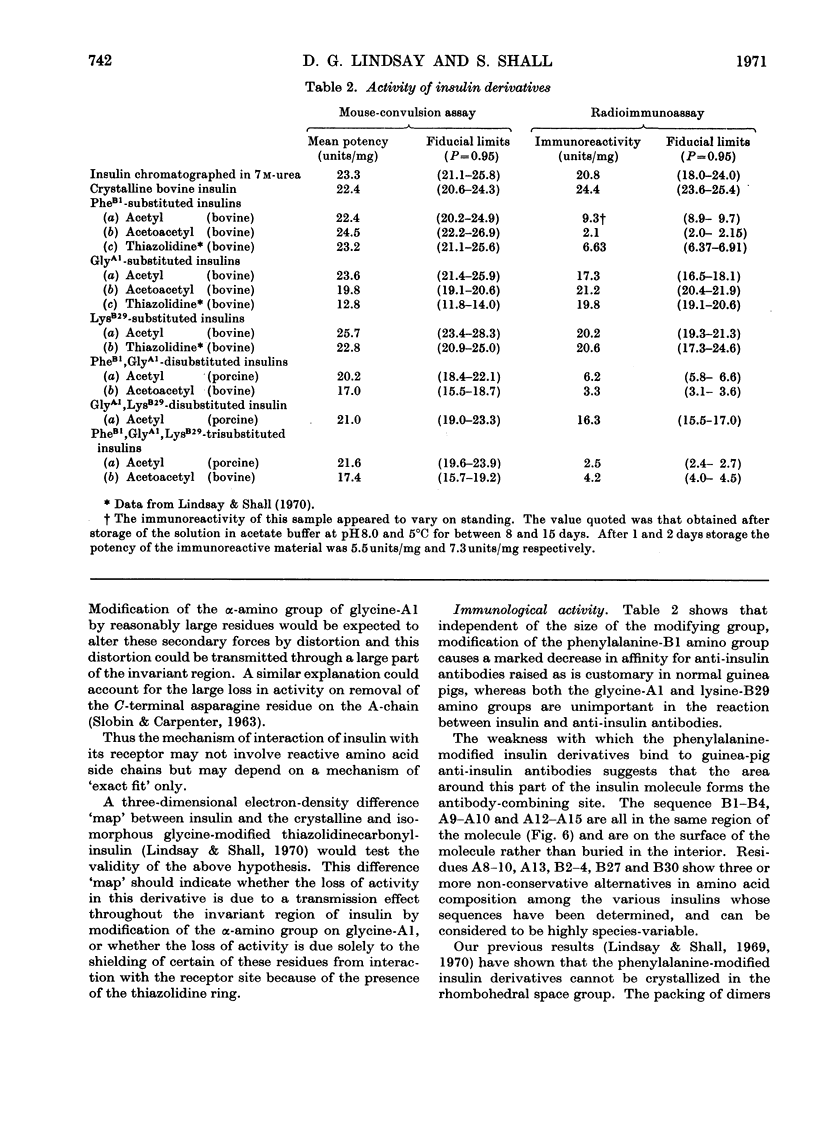
Abstract
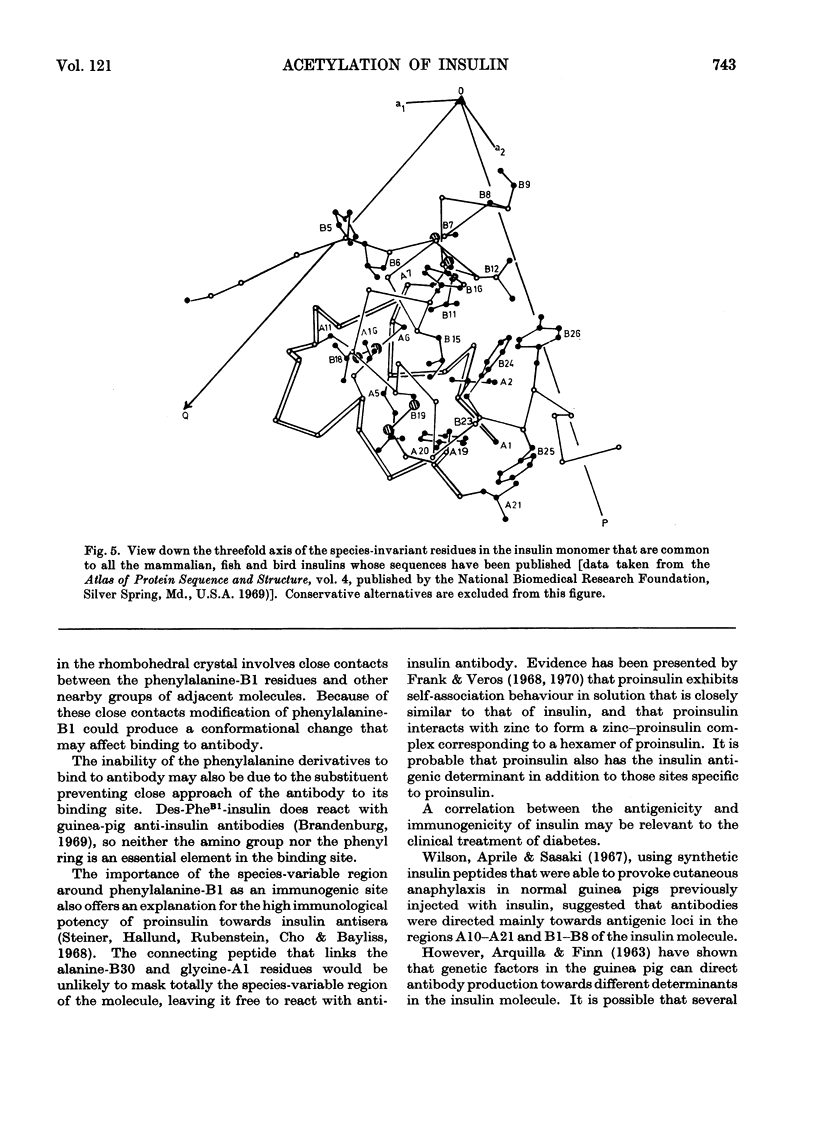
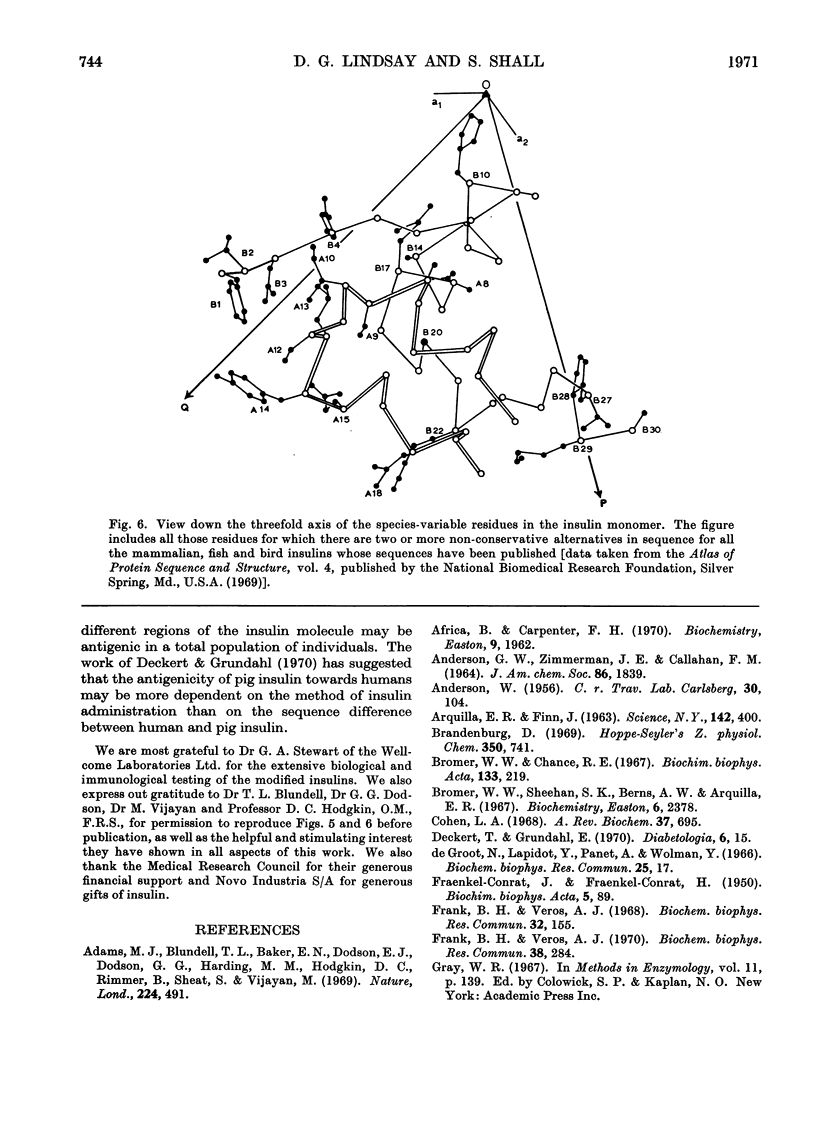
The acetylation of the free amino groups of insulin was studied by reaction of the hormone with N-hydroxysuccinimide acetate at pH6.9 and 8.5. The products formed were separated by chromatography on DEAE-Sephadex and were characterized by isoelectric focusing, by end-group analysis, by the incorporation of [3H]acetyl groups in the molecule, and by treatment with trypsin that had been treated with 1-chloro-4-phenyl-3-toluene-p-sulphonamidobutan-2-one (`tosylphenylalanyl chloromethyl ketone'). Three monosubstituted products, two disubstituted products and one trisubstituted derivative were prepared. The α-amino groups of the terminal residues and the ∈-amino group of the lysine-B29 were the sites of reaction. Acetylation of any of the free amino groups did not affect the biological activity of insulin. It was demonstrated, however, that substitution at the glycine-A1 amino group by the larger residues, acetoacetyl or thiazolidinecarbonyl, produced a decrease in biological activity. Modification of the lysine-B29 or phenylalanine-B1 amino groups with these larger reagents did not affect the biological activity. Modification of the phenylalanine-B1 amino group by any of the three substituents resulted in a large decrease in the affinity of insulin for anti-insulin antibodies raised in the guinea pig. Modification of the other two amino groups did not affect the reaction with antibody. These observations are correlated with the tertiary structure of insulin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARQUILLA E. R., FINN J. GENETIC DIFFERENCES IN ANTIBODY PRODUCTION TO DETERMINANT GROUPS ON INSULIN. Science. 1963 Oct 18;142(3590):400–401. doi: 10.1126/science.142.3590.400. [DOI] [PubMed] [Google Scholar]
- Africa B., Carpenter F. H. Preparation and characterization of diphenylthiocarbamyl-insulin and des-gly A1-des phe B1-insulin (bovine). Biochemistry. 1970 Apr 28;9(9):1962–1972. doi: 10.1021/bi00811a017. [DOI] [PubMed] [Google Scholar]
- Brandenburg D. Des-PheB1-Insulin, ein kristallines Analogon des Rinderinsulins. Hoppe Seylers Z Physiol Chem. 1969 Jun;350(6):741–750. doi: 10.1515/bchm2.1969.350.1.741. [DOI] [PubMed] [Google Scholar]
- Bromer W. W., Chance R. E. Preparation and characterization of desoctapeptide-insulin. Biochim Biophys Acta. 1967 Feb 21;133(2):219–223. doi: 10.1016/0005-2795(67)90061-x. [DOI] [PubMed] [Google Scholar]
- Bromer W. W., Sheehan S. K., Berns A. W., Arquilla E. R. Preparation and properties of fluoresceinthiocarbamyl insulins. Biochemistry. 1967 Aug;6(8):2378–2388. doi: 10.1021/bi00860a013. [DOI] [PubMed] [Google Scholar]
- Deckert T., Grundahl E. The antigenicity of pig insulin. Diabetologia. 1970 Feb;6(1):15–20. doi: 10.1007/BF00425886. [DOI] [PubMed] [Google Scholar]
- Frank B. H., Veros A. J. Interaction of zinc with proinsulin. Biochem Biophys Res Commun. 1970 Jan 23;38(2):284–289. doi: 10.1016/0006-291x(70)90710-2. [DOI] [PubMed] [Google Scholar]
- Frank B. H., Veros A. J. Physical studies on proinsulin-association behavior and conformation in solution. Biochem Biophys Res Commun. 1968 Jul 26;32(2):155–160. doi: 10.1016/0006-291x(68)90362-8. [DOI] [PubMed] [Google Scholar]
- Levy D., Carpenter F. H. The synthesis of triaminoacyl-insulins and the use of the t-butyloxycarbonyl group for the reversible blocking of the amino groups of insulin. Biochemistry. 1967 Nov;6(11):3559–3568. doi: 10.1021/bi00863a030. [DOI] [PubMed] [Google Scholar]
- Lindsay D. G., Shall S. Acetoacetylation of insulin. Biochem J. 1969 Nov;115(3):587–595. doi: 10.1042/bj1150587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay D. G., Shall S. Monosubstituted 2,2-dimethyl-3-formyl-L-thiazolidine-4-carbonyl-insulins. Eur J Biochem. 1970 Sep;15(3):547–554. doi: 10.1111/j.1432-1033.1970.tb01039.x. [DOI] [PubMed] [Google Scholar]
- MILLS G. L. Observations on the composition and activity of partially arylated insulin. Biochem J. 1953 Jan;53(1):37–40. doi: 10.1042/bj0530037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Gorden P., Pastan I. "Big insulin": a new component of plasma insulin detected by immunoassay. Proc Natl Acad Sci U S A. 1968 Sep;61(1):138–145. doi: 10.1073/pnas.61.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. F., Hallund O., Rubenstein A., Cho S., Bayliss C. Isolation and properties of proinsulin, intermediate forms, and other minor components from crystalline bovine insulin. Diabetes. 1968 Dec;17(12):725–736. doi: 10.2337/diab.17.12.725. [DOI] [PubMed] [Google Scholar]
- Wilson S., Aprile M. A., Sasaki L. The antigenic loci of insulin. Can J Biochem. 1967 Jul;45(7):1135–1144. doi: 10.1139/o67-130. [DOI] [PubMed] [Google Scholar]
- Young R. W., Fulhorst H. W. Recovery of S35 radioactivity from protein-bearing polyacrylamide gel. Anal Biochem. 1965 May;11(2):389–391. doi: 10.1016/0003-2697(65)90030-8. [DOI] [PubMed] [Google Scholar]