Abstract
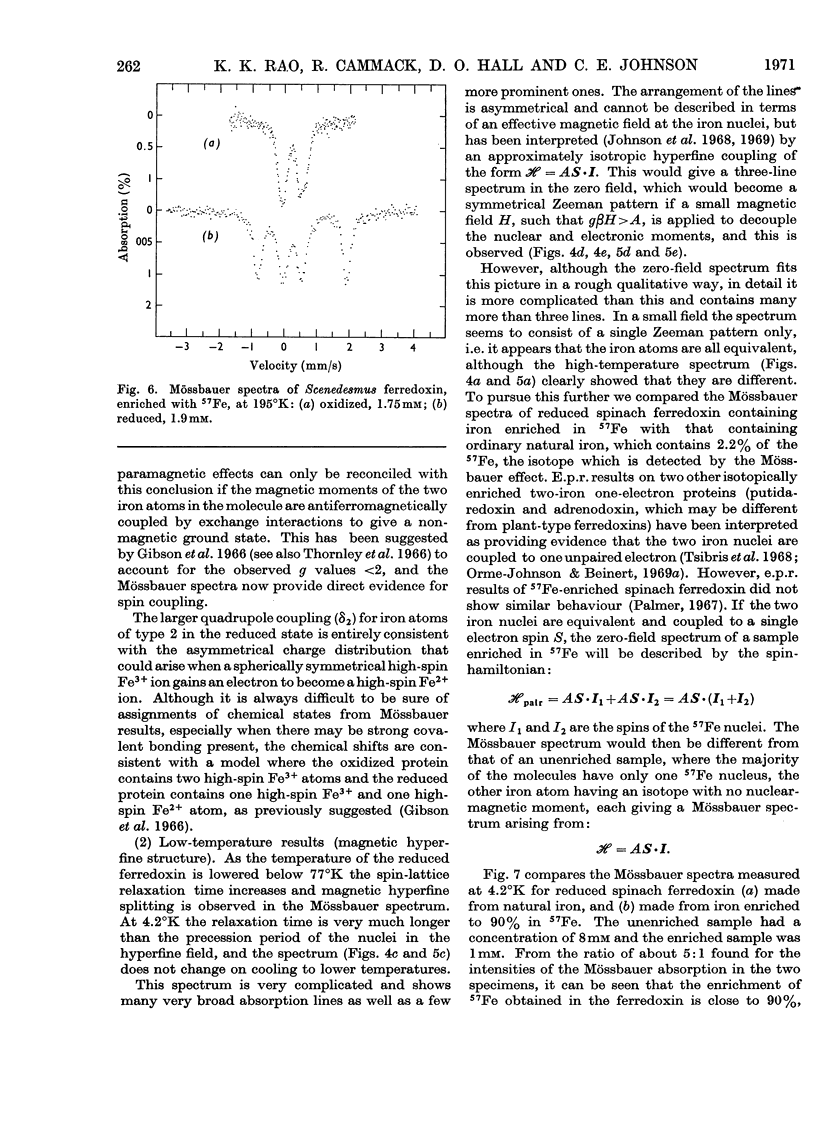
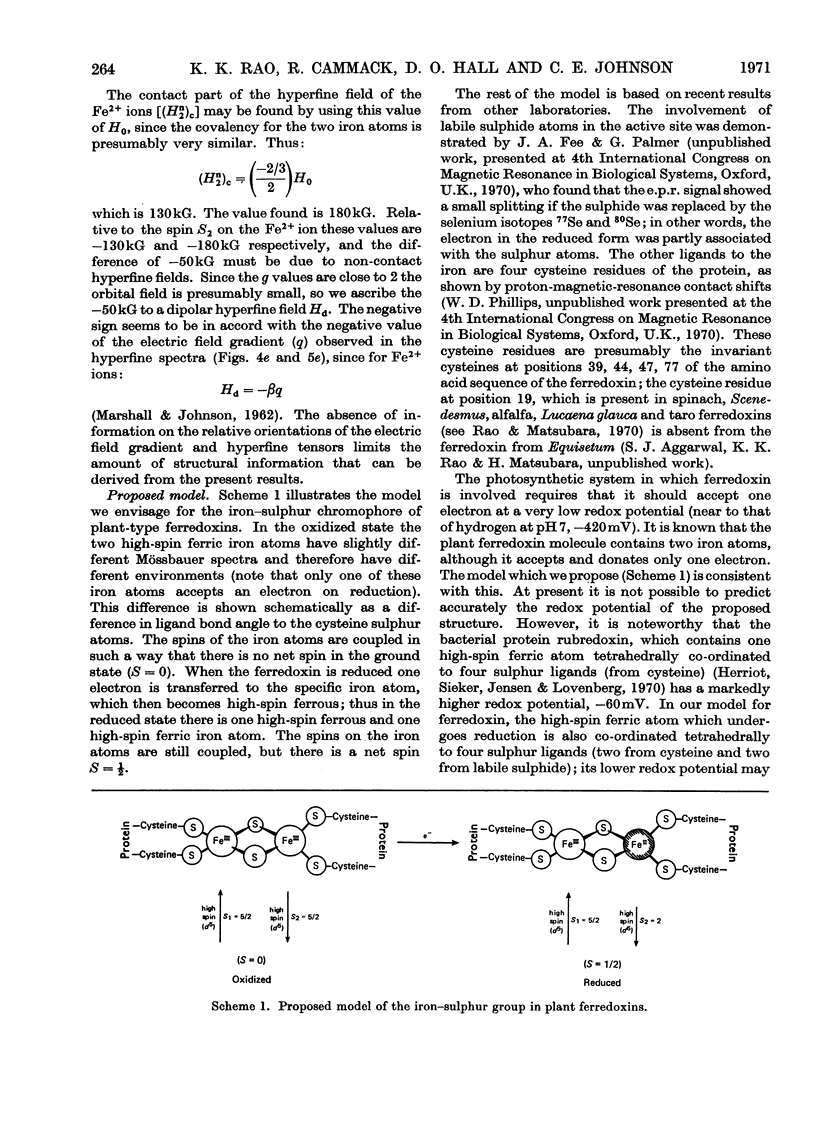
1. The Mössbauer spectra of Scenedesmus ferredoxin enriched in 57Fe were measured and found to be identical with those of two other plant-type ferredoxins (from spinach and Euglena) that had been previously measured. Better resolved Mössbauer spectra of spinach ferredoxin are also reported from protein enriched in 57Fe. All these iron–sulphur proteins are known to contain two iron atoms in a molecule that takes up one electron on reduction. 2. The Mössbauer spectra at 195°K have electric hyperfine structure only and show that on reduction the electron goes to one of the iron atoms, the other appearing to remain unchanged. 3. In the oxidized state, both iron atoms are in a similar chemical state, which appears from the chemical shift and quadrupole splitting to be high-spin Fe3+, but they are in slightly different environments. In the reduced state the iron atoms are different and the molecule appears to contain one high-spin Fe2+ and one high-spin Fe3+ atom. 4. At lower temperatures (77 and 4.2°K) the spectra of both iron atoms in the reduced proteins show magnetic hyperfine structure which suggests that the iron in the oxidized state also has unpaired electrons. This provides experimental evidence for earlier suggestions that in the oxidized state there is antiferromagnetic exchange coupling, which would result in a low value for the magnetic susceptibility. 5. In a small magnetic field the spectrum of the reduced ferredoxin shows a Zeeman splitting with hyperfine field (Hn) of 180kG at the nuclei. On application of a strong magnetic field H the spectrum splits into two spectra with effective fields Hn±H, thus confirming the presence of the two antiferromagnetically coupled iron atoms. 6. These results are in agreement with the model proposed by Gibson, Hall, Thornley & Whatley (1966); in the oxidized state there are two Fe3+ atoms (high spin) antiferromagnetically coupled and on reduction of the ferredoxin by one electron one of the ferric atoms becomes Fe2+ (high spin).
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer E., Josef D., Krauss P., Hagenmaier H., Röder A., Trebst A. Abbau und Resynthese des Aktivzentrums vom Pflanzenferredoxin. Biochim Biophys Acta. 1967 Sep 6;143(2):435–437. doi: 10.1016/0005-2728(67)90100-4. [DOI] [PubMed] [Google Scholar]
- Beinert H., Orme-Johnson W. H. Electron-nuclear and electron-electron spin interactions in the study of enzyme structure and function. Ann N Y Acad Sci. 1969 May 16;158(1):336–360. doi: 10.1111/j.1749-6632.1969.tb56230.x. [DOI] [PubMed] [Google Scholar]
- Garbett K., Gillard R. D., Knowles P. F., Stangroom J. E. Cotton effects in plant ferredoxin and xanthine oxidase. Nature. 1967 Aug 19;215(5103):824–828. doi: 10.1038/215824a0. [DOI] [PubMed] [Google Scholar]
- Gibson J. F., Hall D. O., Thornley J. H., Whatley F. R. The iron complex in spinach ferredoxin. Proc Natl Acad Sci U S A. 1966 Sep;56(3):987–990. doi: 10.1073/pnas.56.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. O., Evans M. C. Iron-sulphur proteins. Nature. 1969 Sep 27;223(5213):1342–1348. doi: 10.1038/2231342a0. [DOI] [PubMed] [Google Scholar]
- Herriott J. R., Sieker L. C., Jensen L. H., Lovenberg W. Structure of rubredoxin: an x-ray study to 2.5 A resolution. J Mol Biol. 1970 Jun 14;50(2):391–406. doi: 10.1016/0022-2836(70)90200-7. [DOI] [PubMed] [Google Scholar]
- Johnson C. E., Bray R. C., Cammack R., Hall D. O. Mossbauer spectroscopy of the iron-sulfur proteins. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1234–1238. doi: 10.1073/pnas.63.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. E., Elstner E., Gibson J. F., Benfield G., Evans M. C., Hall D. O. Mössbauer effect in the ferredoxin of Euglena. Nature. 1968 Dec 28;220(5174):1291–1293. doi: 10.1038/2201291a0. [DOI] [PubMed] [Google Scholar]
- Johnson C. E., Hall D. O. Mössbauer effect study of the state of iron in spinach ferredoxin. Nature. 1968 Feb 3;217(5127):446–448. doi: 10.1038/217446a0. [DOI] [PubMed] [Google Scholar]
- Keresztes-Nagy S., Margoliash E. Preparation and characterization of alfalfa ferredoxin. J Biol Chem. 1966 Dec 25;241(24):5955–5966. [PubMed] [Google Scholar]
- LOVENBERG W., BUCHANAN B. B., RABINOWITZ J. C. STUDIES ON THE CHEMICAL NATURE OF CLOSTRIDIAL FERREDOXIN. J Biol Chem. 1963 Dec;238:3899–3913. [PubMed] [Google Scholar]
- Matsubara H. Puriication and some properties of Scenedesmus ferredoxin. J Biol Chem. 1968 Jan 25;243(2):370–375. [PubMed] [Google Scholar]
- Moss T. H., Bearden A. J., Bartsch R. G., Cusanovich M. A., San Pietro A. Mössbauer spectroscopy of non-heme iron proteins. Biochemistry. 1968 Apr;7(4):1591–1596. doi: 10.1021/bi00844a048. [DOI] [PubMed] [Google Scholar]
- Moss T. H., Petering D., Palmer G. The magnetic susceptibility of oxidized and reduced ferredoxins from spinach and parsley and the high potential protein from Chromatium. J Biol Chem. 1969 May 10;244(9):2275–2277. [PubMed] [Google Scholar]
- Palmer G., Sands R. H. On the magnetic resonance of spinach ferredoxin. J Biol Chem. 1966 Jan 10;241(1):253–253. [PubMed] [Google Scholar]
- Rao K. K., Matsubara H. The amino acid sequence of taro ferredoxin. Biochem Biophys Res Commun. 1970 Feb 6;38(3):500–506. doi: 10.1016/0006-291x(70)90742-4. [DOI] [PubMed] [Google Scholar]
- Thornley J. H., Gibson J. F., Whatley F. R., Hall D. O. Comment on a recent model of the iron complex in spinach ferredoxin. Biochem Biophys Res Commun. 1966 Sep 22;24(6):877–879. doi: 10.1016/0006-291x(66)90330-5. [DOI] [PubMed] [Google Scholar]
- Tsibris J. C., Tsai R. L., Gunsalus I. C., Orme-Johnson W. H., Hansen R. E., Beinert H. The number of iron atoms in the paramagnetic center (G =1.94) of reduced putidaredoxin, a nonheme iron protein. Proc Natl Acad Sci U S A. 1968 Mar;59(3):959–965. doi: 10.1073/pnas.59.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. S., Huennekens F. M. Iron-mercaptoethanol-inorganic sulfide complex. Possible model for the chromophore of nonheme iron proteins. Biochemistry. 1970 May 12;9(10):2127–2133. doi: 10.1021/bi00812a015. [DOI] [PubMed] [Google Scholar]


