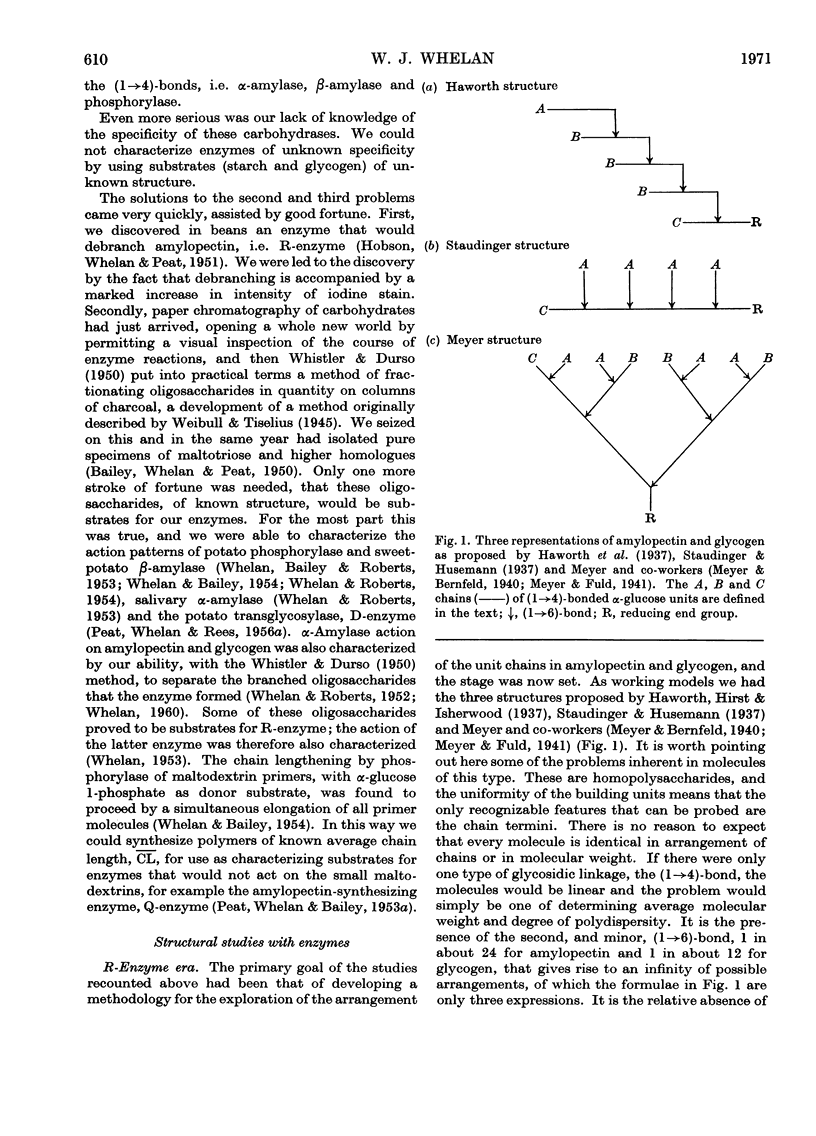
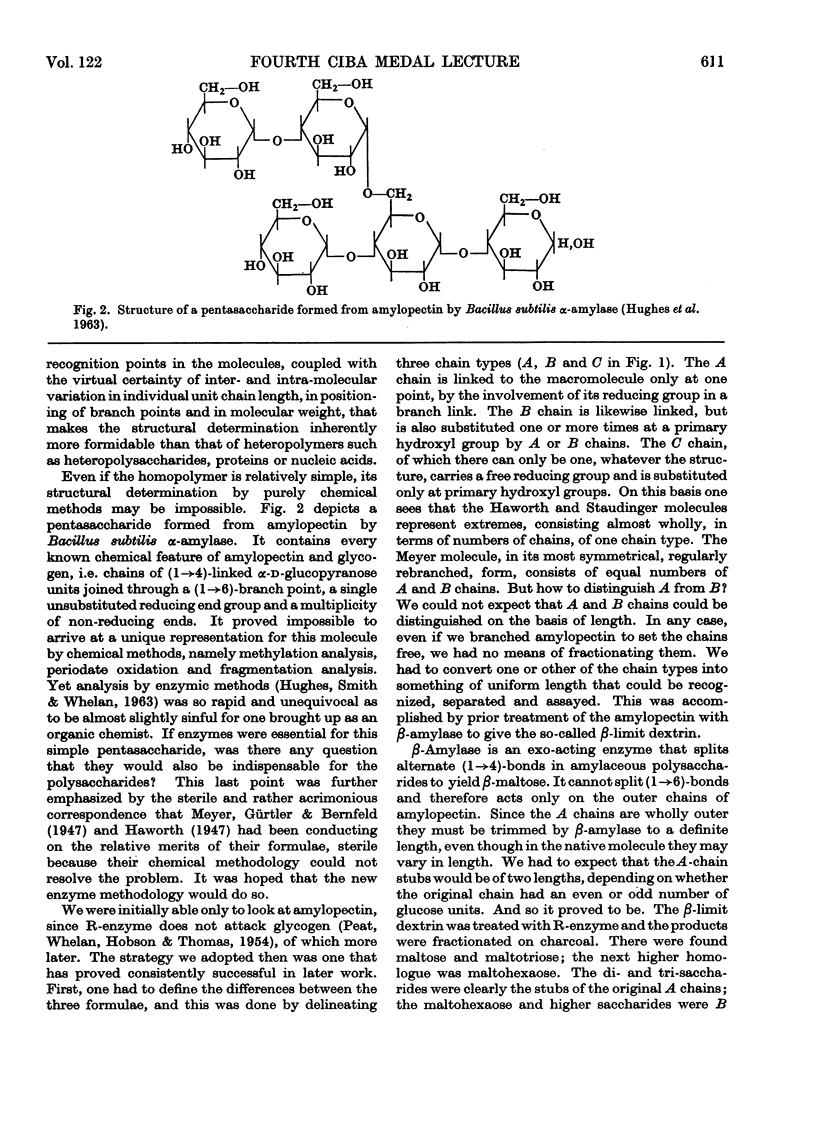
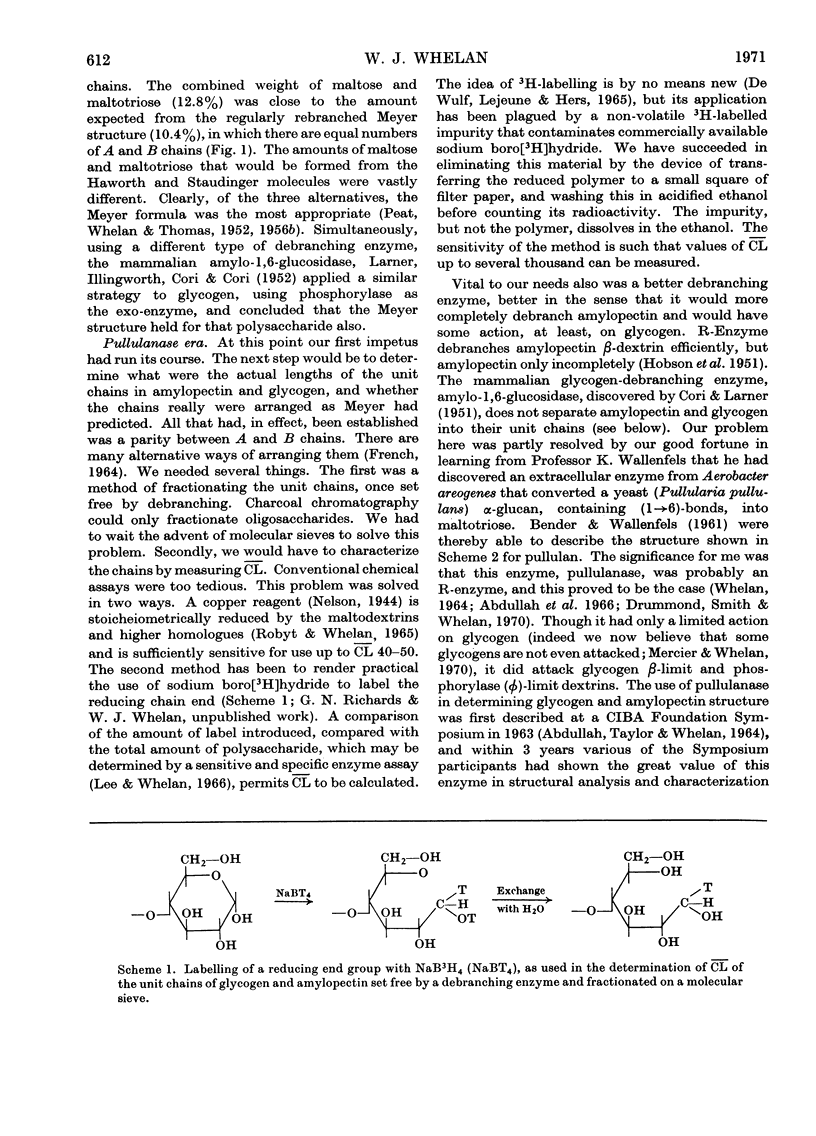
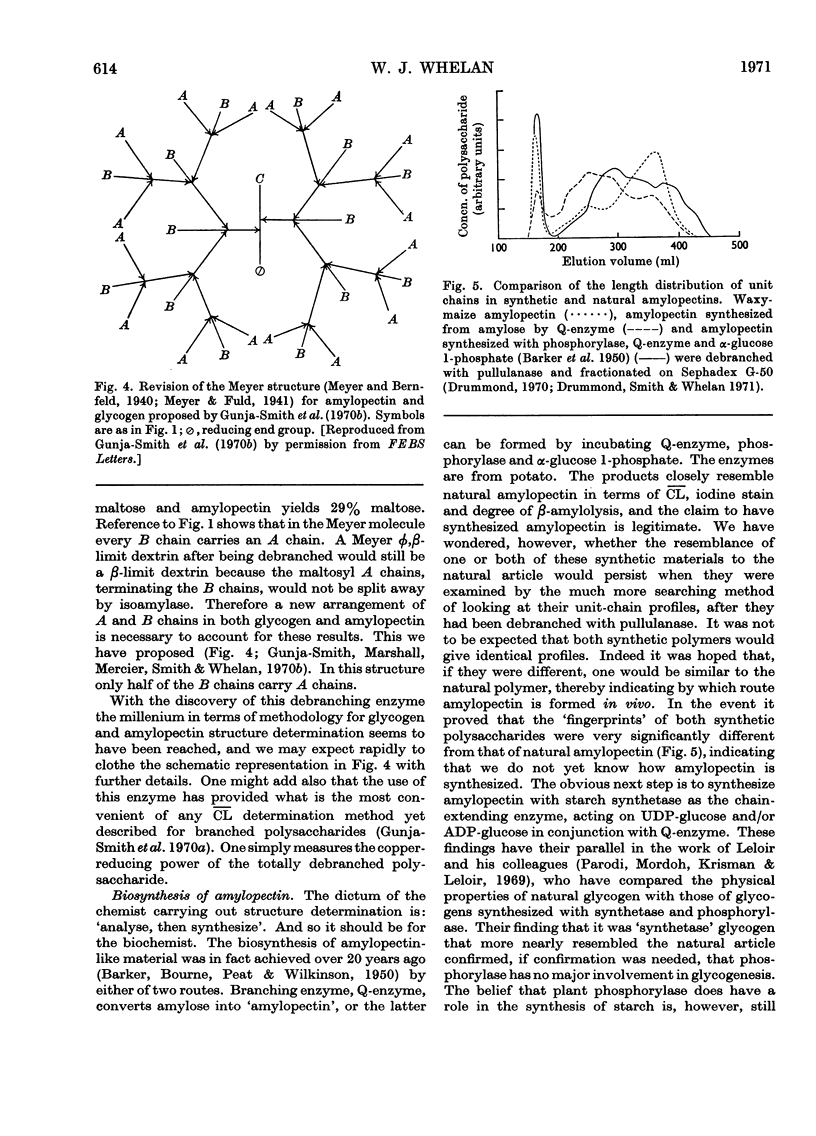
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABDULLAH M., WHELAN W. J. A new pathway in rabbit muscle for the enzymatic debranching of glycogen. Nature. 1963 Mar 9;197:979–980. doi: 10.1038/197979a0. [DOI] [PubMed] [Google Scholar]
- Abdullah M., French D. Reversible action of pullulanase. Nature. 1966 Apr 9;210(5032):200–200. doi: 10.1038/210200a0. [DOI] [PubMed] [Google Scholar]
- Abdullah M., French D. Substrate specificity of pullulanase. Arch Biochem Biophys. 1970 Apr;137(2):483–493. doi: 10.1016/0003-9861(70)90466-2. [DOI] [PubMed] [Google Scholar]
- BROWN D. H., ILLINGWORTH B., CORI C. F. Combined action of oligo-1, 4--1,4-glucantransferase and amylo-1,6-glucosidase in debranching glycogen. Nature. 1963 Mar 9;197:980–982. doi: 10.1038/197980a0. [DOI] [PubMed] [Google Scholar]
- BROWN D. H., ILLINGWORTH B., KORNFELD R. TRANSFER OF GLUCOSYL UNITS TO OLIGOSACCHARIDES AND POLYSACCHARIDES BY THE ACTION OF URIDINE DIPHOSPHOGLUCOSE-ALPHA-GLUCAN TRANSGLUCOSYLASE. Biochemistry. 1965 Mar;4:486–495. doi: 10.1021/bi00879a017. [DOI] [PubMed] [Google Scholar]
- BROWN D. H., ILLINGWORTH B. The properties of an oligo-1,4--1,4-glucantransferase from animal tissues. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1783–1787. doi: 10.1073/pnas.48.10.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. I., Brown D. H. Lack of an alpha-1,4-glucan: alpha-1,4-glucan 6-glycosyl transferase in a case of type IV glycogenosis. Proc Natl Acad Sci U S A. 1966 Aug;56(2):725–729. doi: 10.1073/pnas.56.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. H., Illingworth B., Cori C. F. THE MECHANISM OF THE DE NOVO SYNTHESIS OF POLYSACCHARIDE BY PHOSPHORYLASE. Proc Natl Acad Sci U S A. 1961 Apr;47(4):479–485. doi: 10.1073/pnas.47.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORI G. T., LARNER J. Action of amylo-1,6-glucosidase and phosphorylase on glycogen and amylopectin. J Biol Chem. 1951 Jan;188(1):17–29. [PubMed] [Google Scholar]
- De Wulf H., Lejeune N., Hers H. G. Détermination du poids moléculaire du glycogène par réaction de son extrémité réductrice avec le borohydrure tritié. Arch Int Physiol Biochim. 1965 Mar;73(2):362–364. [PubMed] [Google Scholar]
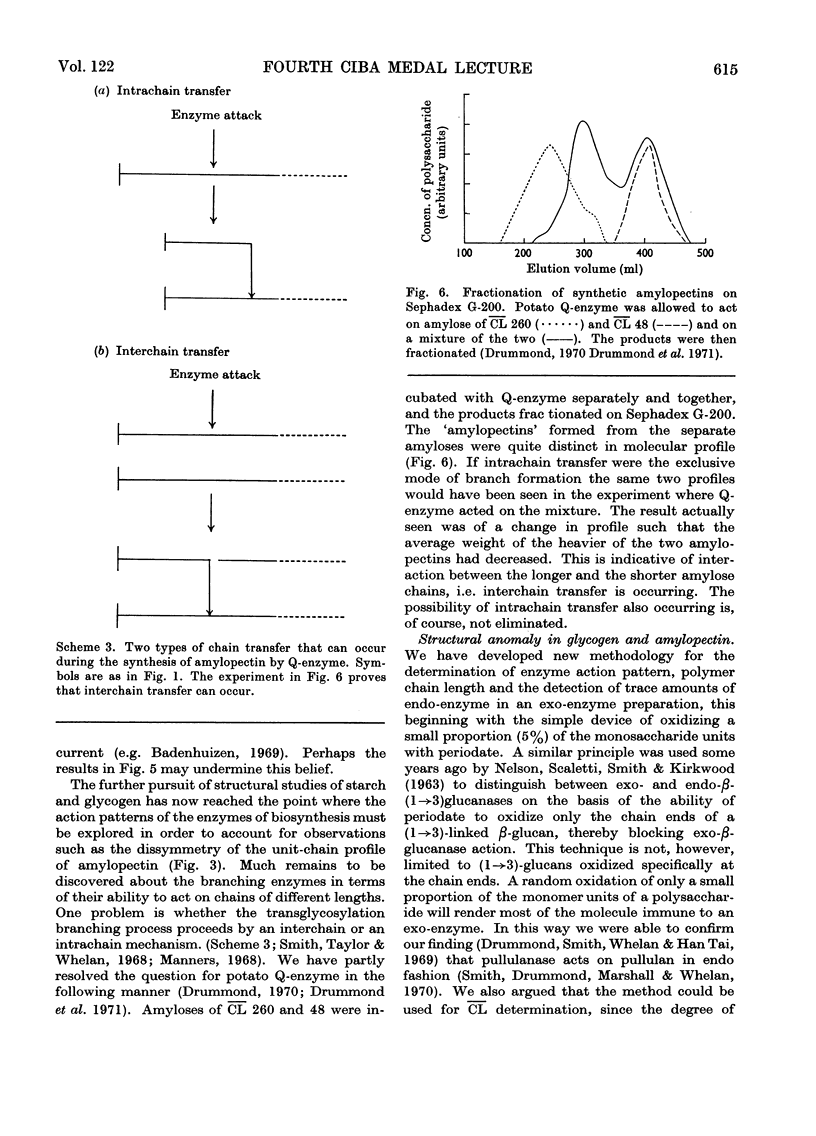
- Drummond G. S., Smith E. E., Whelan W. J. On the specificity of starch debranching enzymes. FEBS Lett. 1970 Sep 6;9(3):136–140. doi: 10.1016/0014-5793(70)80337-4. [DOI] [PubMed] [Google Scholar]
- GOLDEMBERG S. H. Specificity of uridine diphosphate glucose-glycogen glucosyltransferase. Biochim Biophys Acta. 1962 Jan 29;56:357–359. doi: 10.1016/0006-3002(62)90576-0. [DOI] [PubMed] [Google Scholar]
- Gunja-Smith Z., Marshall J. J., Mercier C., Smith E. E., Whelan W. J. A revision of the Meyer-Bernfeld model of glycogen and amylopectin. FEBS Lett. 1970 Dec 28;12(2):101–104. doi: 10.1016/0014-5793(70)80573-7. [DOI] [PubMed] [Google Scholar]
- Gunja-Smith Z., Marshall J. J., Smith E. E., Whelan W. J. A glycogen-debranching enzyme from Cytophaga. FEBS Lett. 1970 Dec 28;12(2):96–100. doi: 10.1016/0014-5793(70)80572-5. [DOI] [PubMed] [Google Scholar]
- Hers H. G., Verhue W., Van hoof F. The determination of amylo-1,6-glucosidase. Eur J Biochem. 1967 Oct;2(3):257–264. doi: 10.1111/j.1432-1033.1967.tb00133.x. [DOI] [PubMed] [Google Scholar]
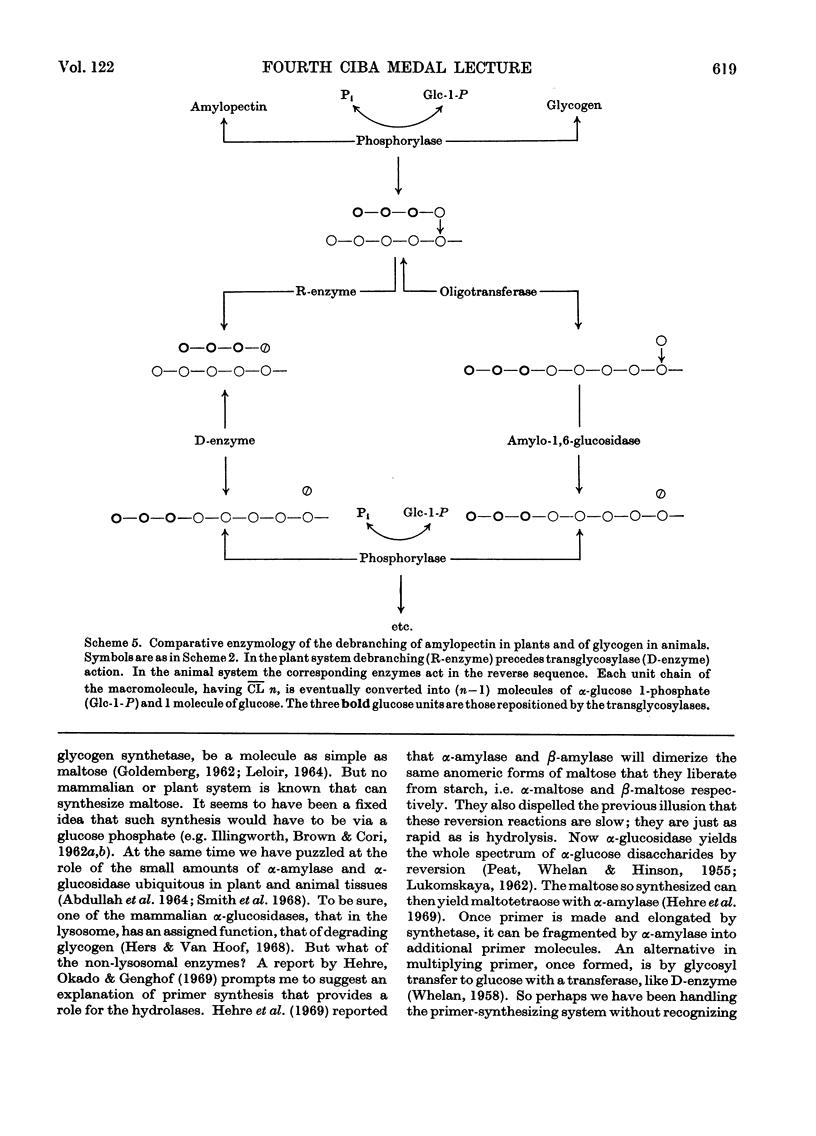
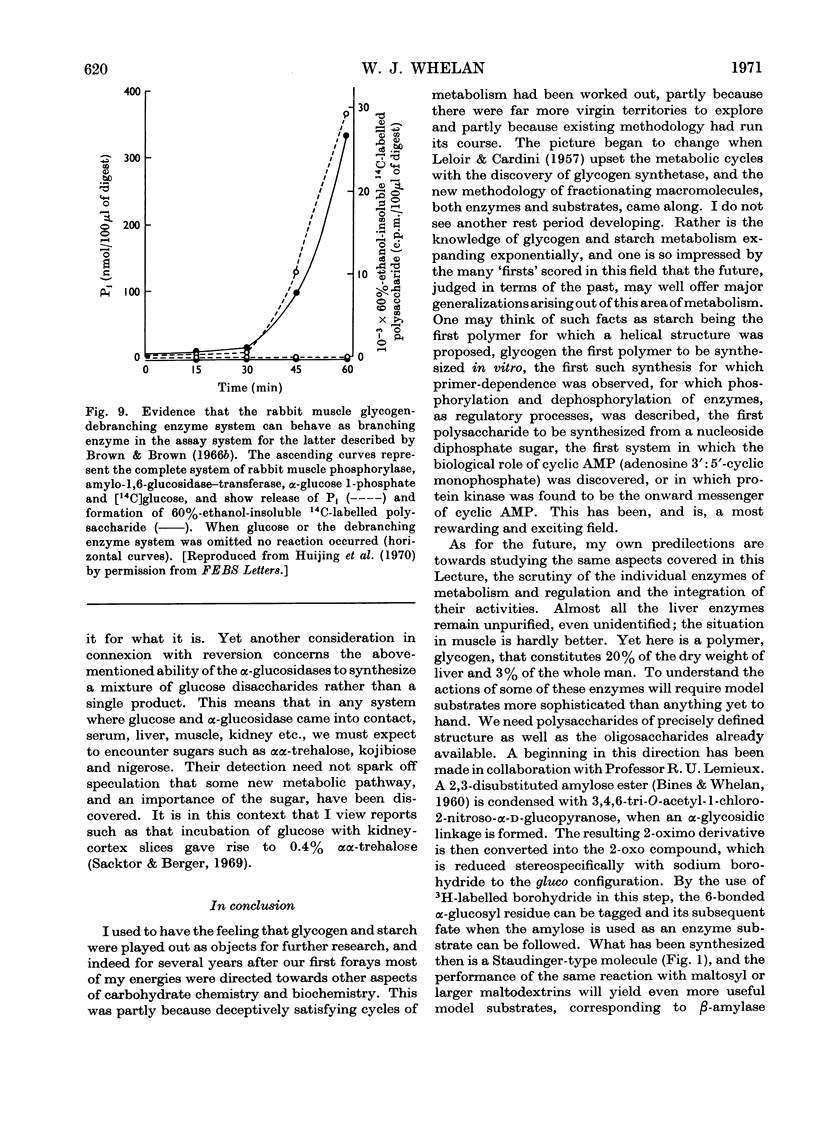
- Huijing F., Lee E. Y.C., Carter J. H., Whelan W. J. Branching action of amylo-1,6-glucosidase/oligo-1,4-->1,4-glucantransferase. FEBS Lett. 1970 Apr 16;7(3):251–254. doi: 10.1016/0014-5793(70)80173-9. [DOI] [PubMed] [Google Scholar]
- ILLINGWORTH B., BROWN D. H., CORI C. F. The de novo synthesis of polysaccharide by phosphorylase. Proc Natl Acad Sci U S A. 1961 Apr 15;47:469–478. doi: 10.1073/pnas.47.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ILLINGWORTH B., LARNER J., CORI G. T. Structure of glycogens and amylopectins. I. Enzymatic determination of chain length. J Biol Chem. 1952 Dec;199(2):631–640. [PubMed] [Google Scholar]
- LARNER J., ILLINGWORTH B., CORI G. T., CORI C. F. Structure of glycogens and amylopectins. II. Analysis by stepwise enzymatic degradation. J Biol Chem. 1952 Dec;199(2):641–651. [PubMed] [Google Scholar]
- LUKOMSKAYA I. S. The synthesis of oligosaccharides with different types of linkage in animal tissues. Enzymologia. 1962 Aug 15;24:327–337. [PubMed] [Google Scholar]
- Lee E. Y., Carter J. H., Nielsen L. D., Fischer E. H. Purification and properties of yeast amylo-1,6-glucosidase--oligo-1,4 leads to 1,4-glucantransferase. Biochemistry. 1970 May 26;9(11):2347–2355. doi: 10.1021/bi00813a019. [DOI] [PubMed] [Google Scholar]
- Lee E. Y., Mercier C., Whelan W. J. A method for the investigation of the fine structure of amylopectin. Arch Biochem Biophys. 1968 Jun;125(3):1028–1030. doi: 10.1016/0003-9861(68)90544-4. [DOI] [PubMed] [Google Scholar]
- Lee E. Y., Whelan W. J. Enzymic methods for the microdetermination of glycogen and amylopectin, and their unit-chain lengths. Arch Biochem Biophys. 1966 Sep 26;116(1):162–167. doi: 10.1016/0003-9861(66)90024-5. [DOI] [PubMed] [Google Scholar]
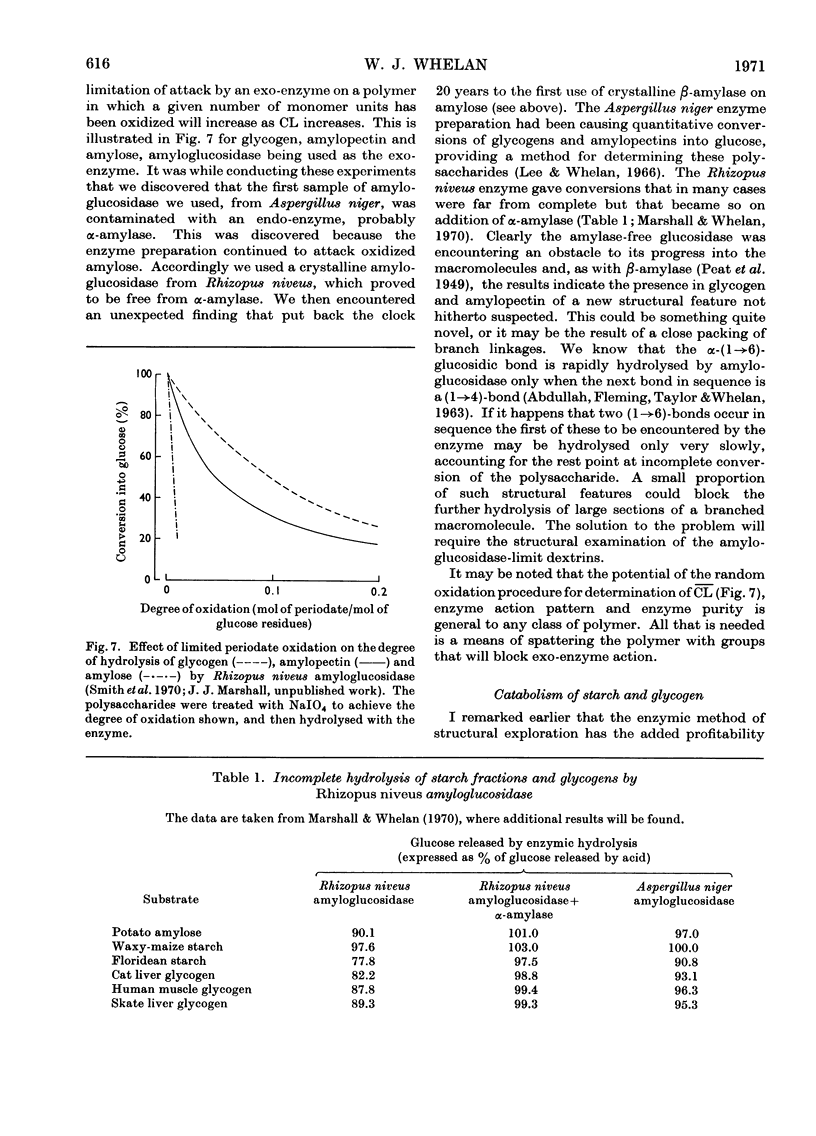
- Marshall J. J., Whelan W. J. Incomplete conversion of glycogen and starch by crystalline amyloglucosidase and its importance in the determination of amylaceous polymers. FEBS Lett. 1970 Jul 29;9(2):85–88. doi: 10.1016/0014-5793(70)80319-2. [DOI] [PubMed] [Google Scholar]
- Mercier C., Whelan W. J. The fine structure of glycogen from type IV glycogen-storage disease. Eur J Biochem. 1970 Nov;16(3):579–583. doi: 10.1111/j.1432-1033.1970.tb01120.x. [DOI] [PubMed] [Google Scholar]
- Mirsky A. E. The discovery of DNA. Sci Am. 1968 Jun;218(6):78–88. doi: 10.1038/scientificamerican0668-78. [DOI] [PubMed] [Google Scholar]
- PEAT S., WHELAN W. J., REES W. R. D-enzyme: a disproportionating enzyme in potato juice. Nature. 1953 Jul 25;172(4369):158–158. doi: 10.1038/172158a0. [DOI] [PubMed] [Google Scholar]
- Parodi A. J., Mordoh J., Krisman C. R., Leloir L. F. In vitro synthesis of particulate glycogen from uridine diphosphate glucose. Arch Biochem Biophys. 1969 Jun;132(1):111–117. doi: 10.1016/0003-9861(69)90342-7. [DOI] [PubMed] [Google Scholar]
- Sacktor B., Berger S. J. Formation of trehalose from glucose in the renal cortex. Biochem Biophys Res Commun. 1969 Jun 27;35(6):796–800. doi: 10.1016/0006-291x(69)90693-7. [DOI] [PubMed] [Google Scholar]
- Verhue W., Hers H. G. A study of the reaction catalysed by the liver branching enzyme. Biochem J. 1966 Apr;99(1):222–227. doi: 10.1042/bj0990222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER G. J., WHELAN W. J. The mechanism of carbohydrase action. 4. The mechanism of D-enzyme action. Biochem J. 1957 Dec;67(4):548–551. doi: 10.1042/bj0670548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER G. J., WHELAN W. J. The mechanism of carbohydrase action. 8. Structures of the muscle-phosphorylase limit dextrins of glycogen and amylopectin. Biochem J. 1960 Aug;76:264–268. doi: 10.1042/bj0760264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHELAN W. J., BAILEY J. M. The action pattern of potato phosphorylase. Biochem J. 1954 Dec;58(4):560–569. doi: 10.1042/bj0580560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHELAN W. J., ROBERTS J. G. Products of the beta-amylolysis of maltodextrins. Biochem J. 1954 Dec;58(4):569–570. doi: 10.1042/bj0580569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHELAN W. J., ROBERTS P. J. P. Action of salivary alpha-amylase on amylopectin and glycogen. Nature. 1952 Nov 1;170(4331):748–749. doi: 10.1038/170748a0. [DOI] [PubMed] [Google Scholar]
- Yokobayashi K., Misaki A., Harada T. Purification and properties of Pseudomonas isoamylase. Biochim Biophys Acta. 1970 Sep 16;212(3):458–469. doi: 10.1016/0005-2744(70)90252-4. [DOI] [PubMed] [Google Scholar]



