Abstract
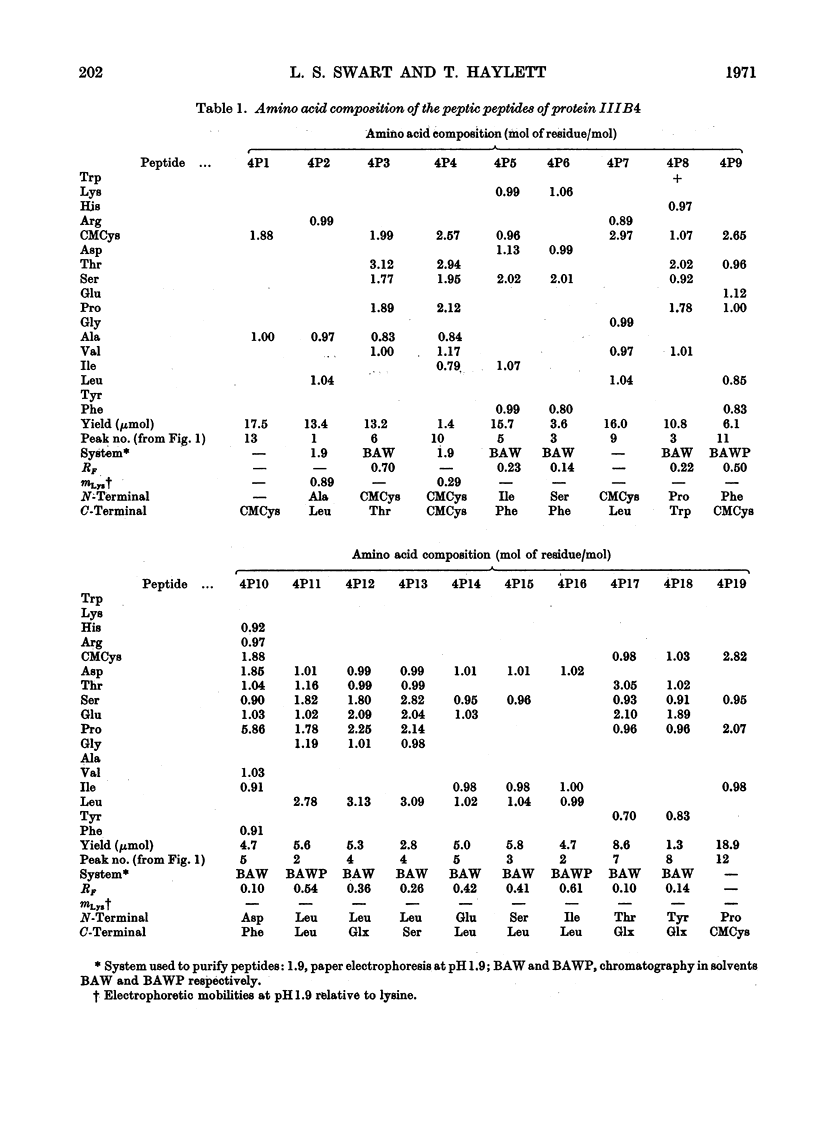
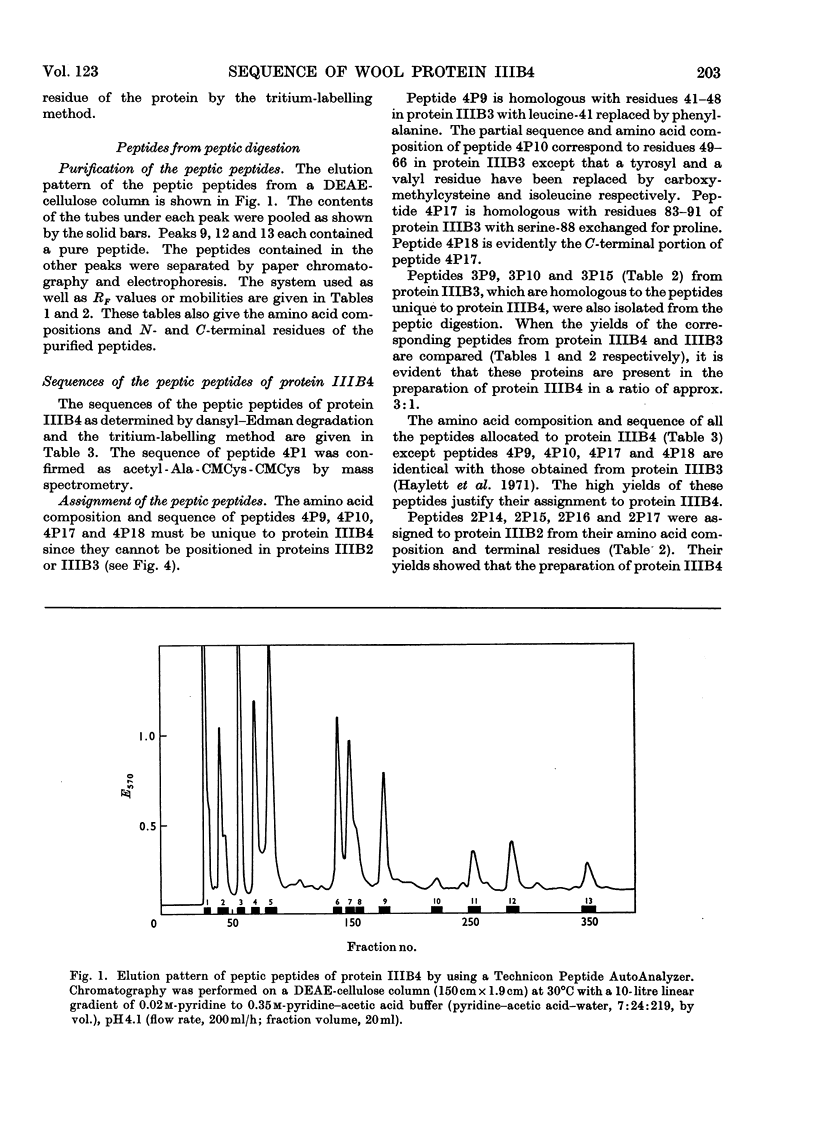
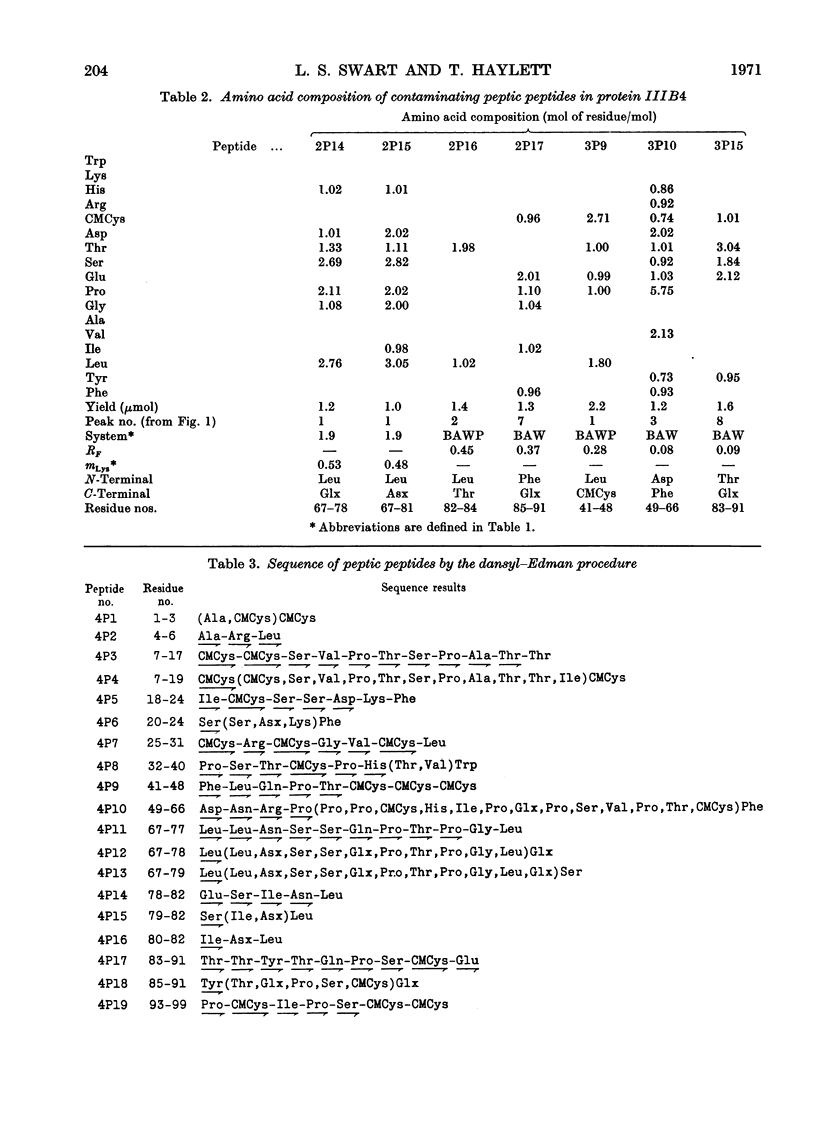
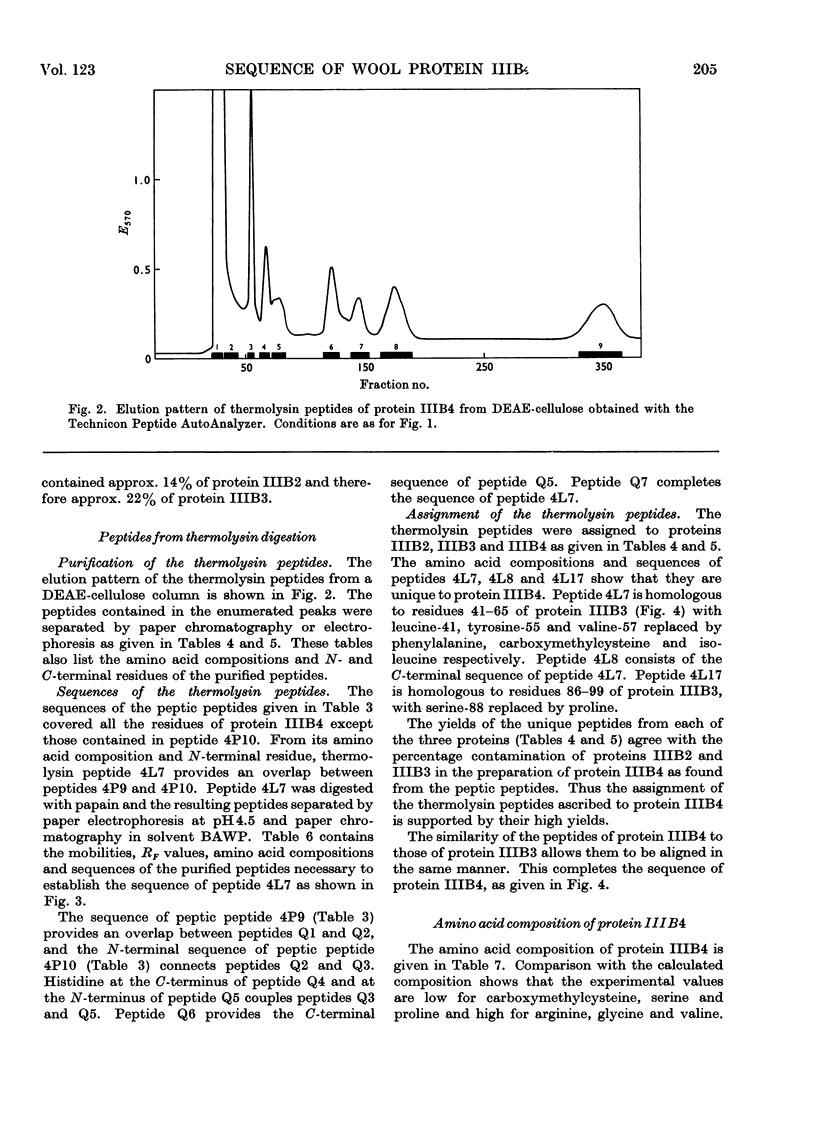
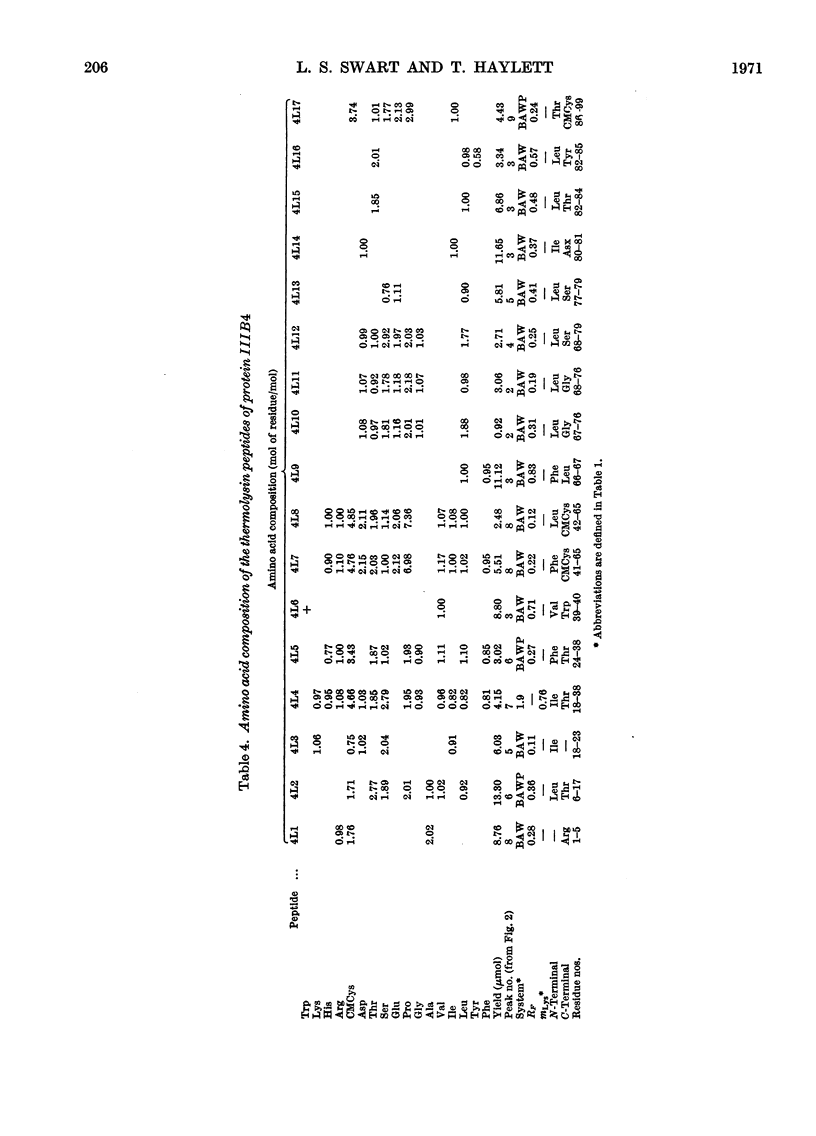
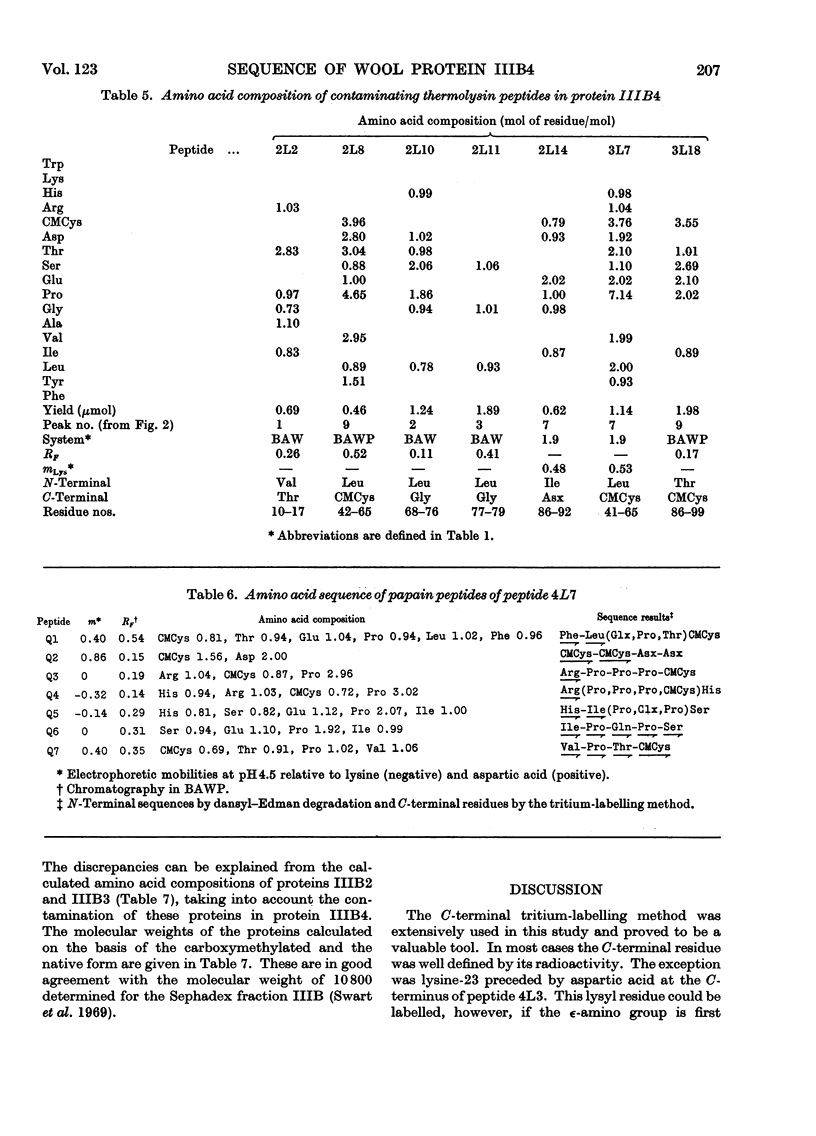
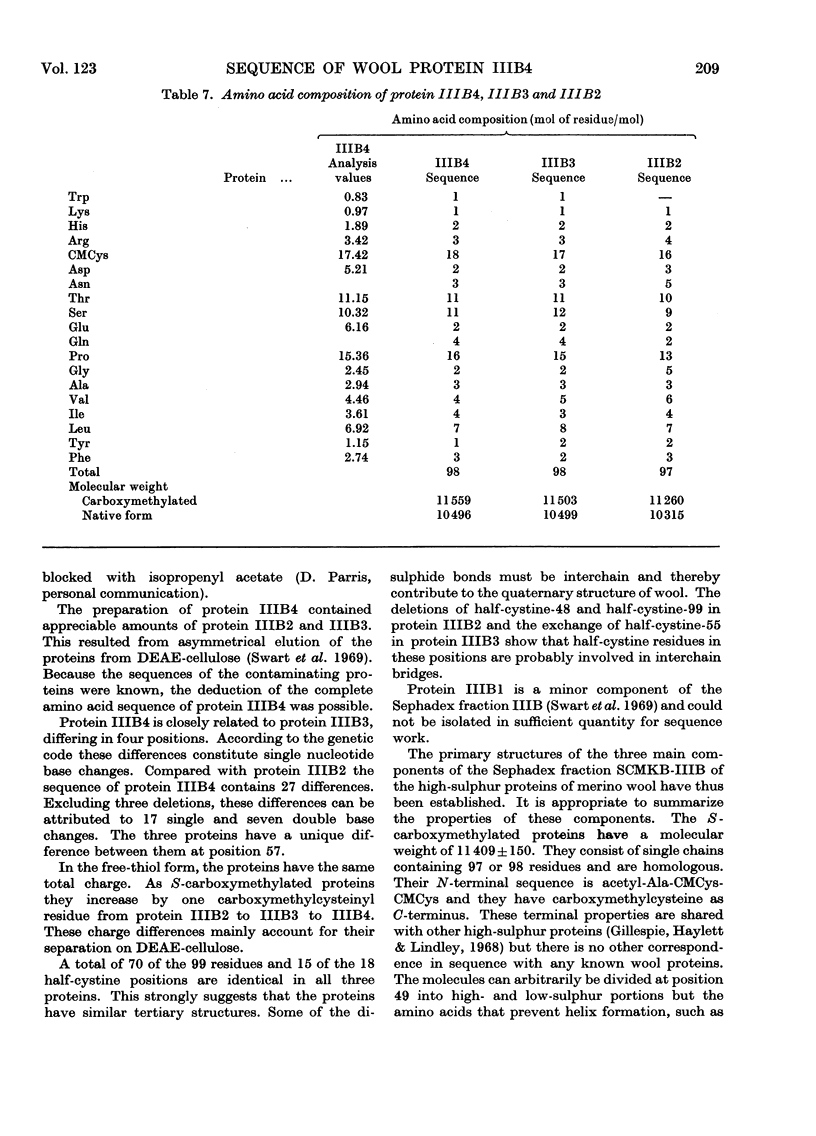
The complete amino acid sequence of protein SCMKB-IIIB4 is presented. It is closely related to the sequence of protein SCMKB-IIIB3 (Haylett, Swart & Parris, 1971) differing in only four positions. The peptic and thermolysin peptides of protein SCMKB-IIIB4 were analysed by the dansyl–Edman method (Gray, 1967) and by tritium-labelling of C-terminal residues (Matsuo, Fujimoto & Tatsuno, 1966). This protein is the third member of a group of high-sulphur wool proteins with molecular weight of about 11400. It consists of 98 residues and has acetylalanine and carboxymethylcysteine as N- and C-terminal residues respectively.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gillespie J. M., Haylett T., Lindley H. Evidence of homology in a high-sulphur protein fraction (SCMK-B2) of wool and hair alpha-keratins. Biochem J. 1968 Nov;110(2):193–200. doi: 10.1042/bj1100193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haylett T., Swart L. S., Parris D. Studies on the high-sulphur proteins of reduced merino wool. Amino acid sequence of protein SCMKB-3B 3 . Biochem J. 1971 Jun;123(2):191–200. doi: 10.1042/bj1230191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiryushkin A. A., Gorlenko V. A., Rosinov B. V., Ovchinnikov Y. A., Shemyakin M. M. Mass spectrometric determination of the amino acid sequence in peptides: desulfurization of sulfur-containing peptides. Experientia. 1969 Sep 15;25(9):913–914. doi: 10.1007/BF01898058. [DOI] [PubMed] [Google Scholar]
- Lenard J., Gallop P. M. Sequence analysis of microgram amounts of peptides by mass spectrometry. Anal Biochem. 1970 Mar;34:286–291. doi: 10.1016/0003-2697(70)90109-0. [DOI] [PubMed] [Google Scholar]


