Abstract
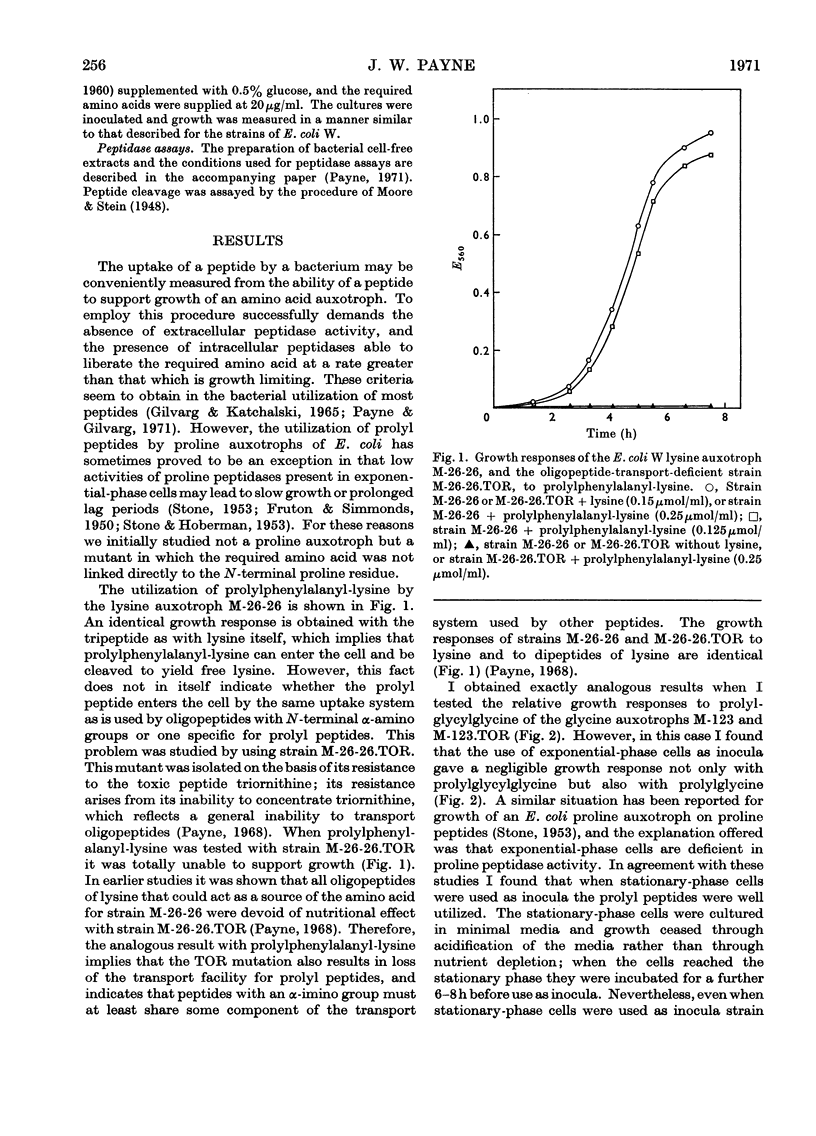
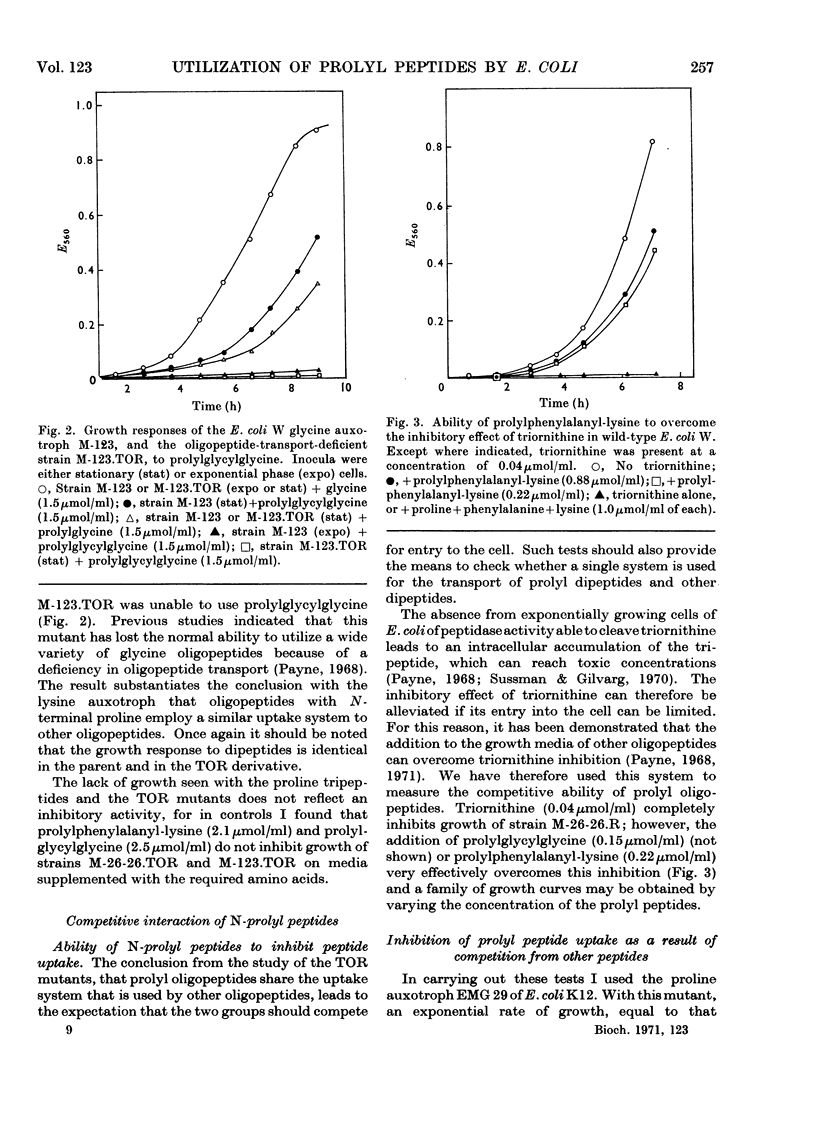
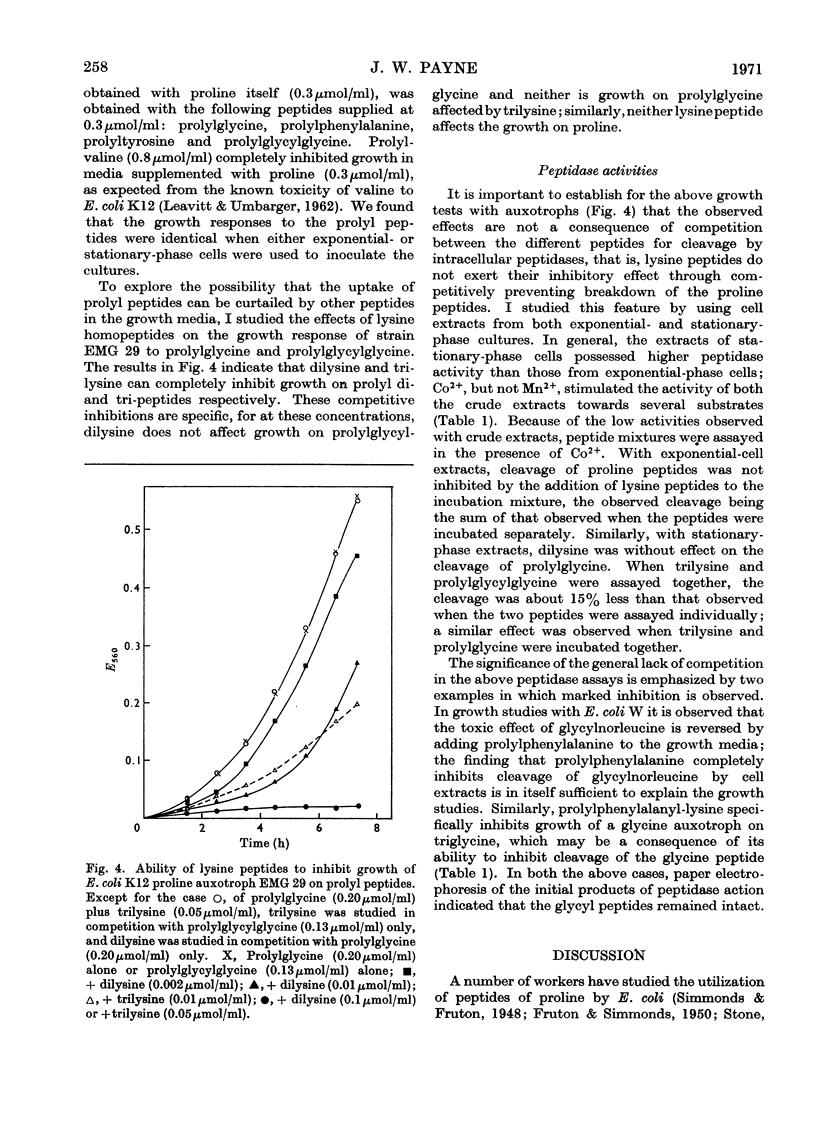
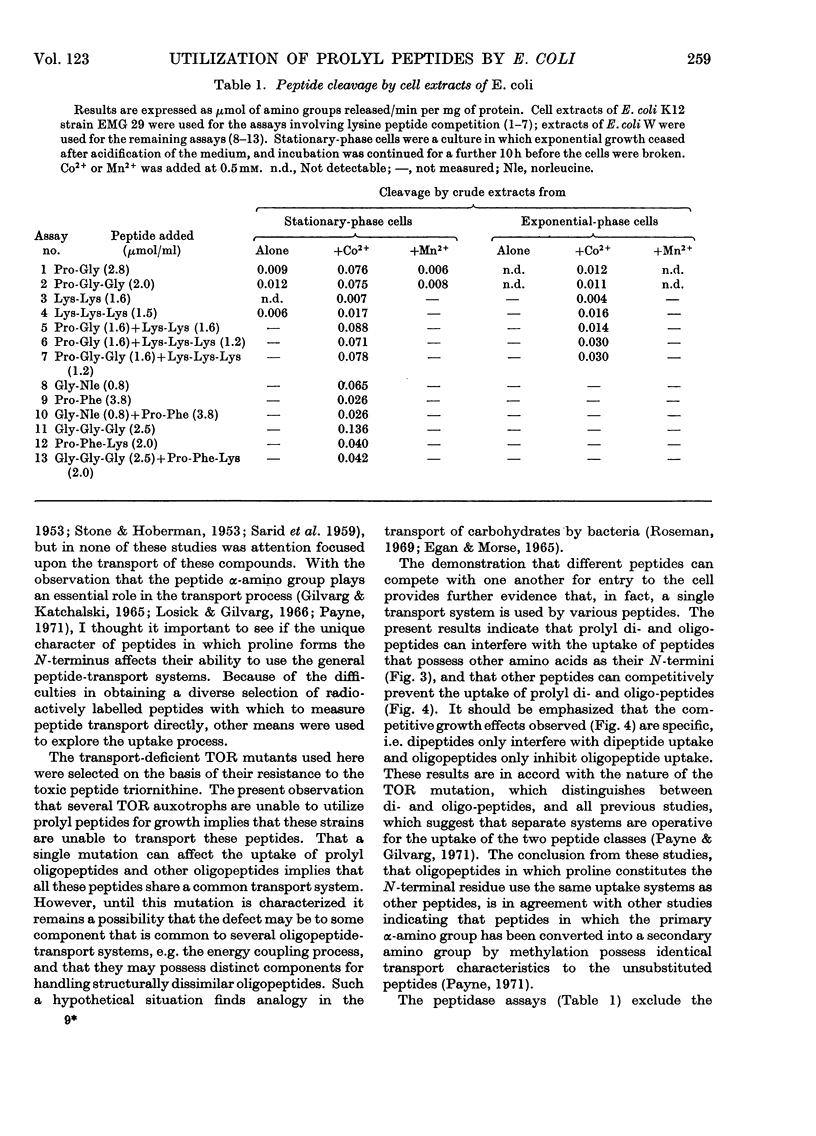
Peptides that have an N-terminal proline residue are taken up by Escherichia coli and are degraded by intracellular peptidases. A mutant that is unable to transport oligopeptides with N-terminal α-amino acids is also unable to transport the peptides with N-terminal proline. Dipeptides and oligopeptides can prevent the uptake of the corresponding prolyl peptides and the converse competitive interactions are also observed. Although the peptide α-amino group is essential to the process of peptide transport, the results with the prolyl peptides indicate that the dipeptide and oligopeptide permeases can handle peptides with either an α-amino or α-imino group.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRITTEN R. J., McCLURE F. T. The amino acid pool in Escherichia coli. Bacteriol Rev. 1962 Sep;26:292–335. doi: 10.1128/br.26.3.292-335.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D. Biosynthetic interrelations of lysine, diaminopimelic acid, and threonine in mutants of Escherichia coli. Nature. 1952 Mar 29;169(4300):534–536. doi: 10.1038/169534a0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEWEY D. L., WORK E. Diaminopimelic acid decarboxylase. Nature. 1952 Mar 29;169(4300):533–534. doi: 10.1038/169533a0. [DOI] [PubMed] [Google Scholar]
- Egan J. B., Morse M. L. Carbohydrate transport in Staphylococcus aureus. II. Characterization of the defect of a pleiotropic transport mutant. Biochim Biophys Acta. 1965 Sep 27;109(1):172–183. doi: 10.1016/0926-6585(65)90101-9. [DOI] [PubMed] [Google Scholar]
- FRUTON J. S., SIMMONDS S. The metabolism of peptides. Cold Spring Harb Symp Quant Biol. 1950;14:55–64. doi: 10.1101/sqb.1950.014.01.009. [DOI] [PubMed] [Google Scholar]
- GILVARG C., KATCHALSKI E. PEPTIDE UTILIZATION IN ESCHERICHIA COLI. J Biol Chem. 1965 Jul;240:3093–3098. [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- LEAVITT R. I., UMBARGER H. E. Isoleucine and valine metabolism in Escherichia coli. XI. Valine inhibition of the growth of Escherichia coli strain K-12. J Bacteriol. 1962 Mar;83:624–630. doi: 10.1128/jb.83.3.624-630.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R., Gilvarg C. Effect of alpha-acetylation on utilization of lysine oligopeptides in Escherichia coli. J Biol Chem. 1966 May 25;241(10):2340–2346. [PubMed] [Google Scholar]
- OXENDER D. L., CHRISTENSEN H. N. DISTINCT MEDIATING SYSTEMS FOR THE TRANSPORT OF NEUTRAL AMINO ACIDS BY THE EHRLICH CELL. J Biol Chem. 1963 Nov;238:3686–3699. [PubMed] [Google Scholar]
- Payne J. W. Oligopeptide transport in Escherichia coli. Specificity with respect to side chain and distinction from dipeptide transport. J Biol Chem. 1968 Jun 25;243(12):3395–3403. [PubMed] [Google Scholar]
- Payne J. W. The requirement for the protonated -amino group for the transport of peptides in Escherichia coli. Biochem J. 1971 Jun;123(2):245–253. doi: 10.1042/bj1230245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARID S., BERGER A., KATCHALSKI E. Proline iminopeptidase. II. Purification and comparison with iminodipeptidase (prolinase). J Biol Chem. 1962 Jul;237:2207–2212. [PubMed] [Google Scholar]
- SARID S., BERGER A., KATCHALSKI E. Proline iminopeptidase. J Biol Chem. 1959 Jul;234(7):1740–1746. [PubMed] [Google Scholar]
- STONE D., HOBERMAN H. D. Utilization of proline peptides by a prolineless mutant of Escherichia coli. J Biol Chem. 1953 May;202(1):203–212. [PubMed] [Google Scholar]
- STONE D. Some aspects of the hydrolysis of proline peptides by a prolineless mutant of Escherichia coli. J Biol Chem. 1953 Jun;202(2):821–827. [PubMed] [Google Scholar]
- Sussman A. J., Gilvarg C. Peptidases in Escherichia coli K-12 capable of cleaving lysine homopeptides. J Biol Chem. 1970 Dec 25;245(24):6518–6524. [PubMed] [Google Scholar]
- Tristram H., Neale S. The activity and specificity of the proline permease in wild-type and analogue-resistant strains of Escherichia coli. J Gen Microbiol. 1968 Jan;50(1):121–137. doi: 10.1099/00221287-50-1-121. [DOI] [PubMed] [Google Scholar]
- Vogt V. M. Purification and properties of an aminopeptidase from Escherichia coli. J Biol Chem. 1970 Sep 25;245(18):4760–4769. [PubMed] [Google Scholar]
- WIESMEYER H., COHN M. The characterization of the pathway of maltose utilization by Escherichia coli. I. Purification and physical chemical properties of the enzyme amylomaltase. Biochim Biophys Acta. 1960 Apr 22;39:417–426. doi: 10.1016/0006-3002(60)90194-3. [DOI] [PubMed] [Google Scholar]
- Yaron A., Mlynar D. Aminopeptidase-P. Biochem Biophys Res Commun. 1968 Aug 21;32(4):658–663. doi: 10.1016/0006-291x(68)90289-1. [DOI] [PubMed] [Google Scholar]


