Abstract
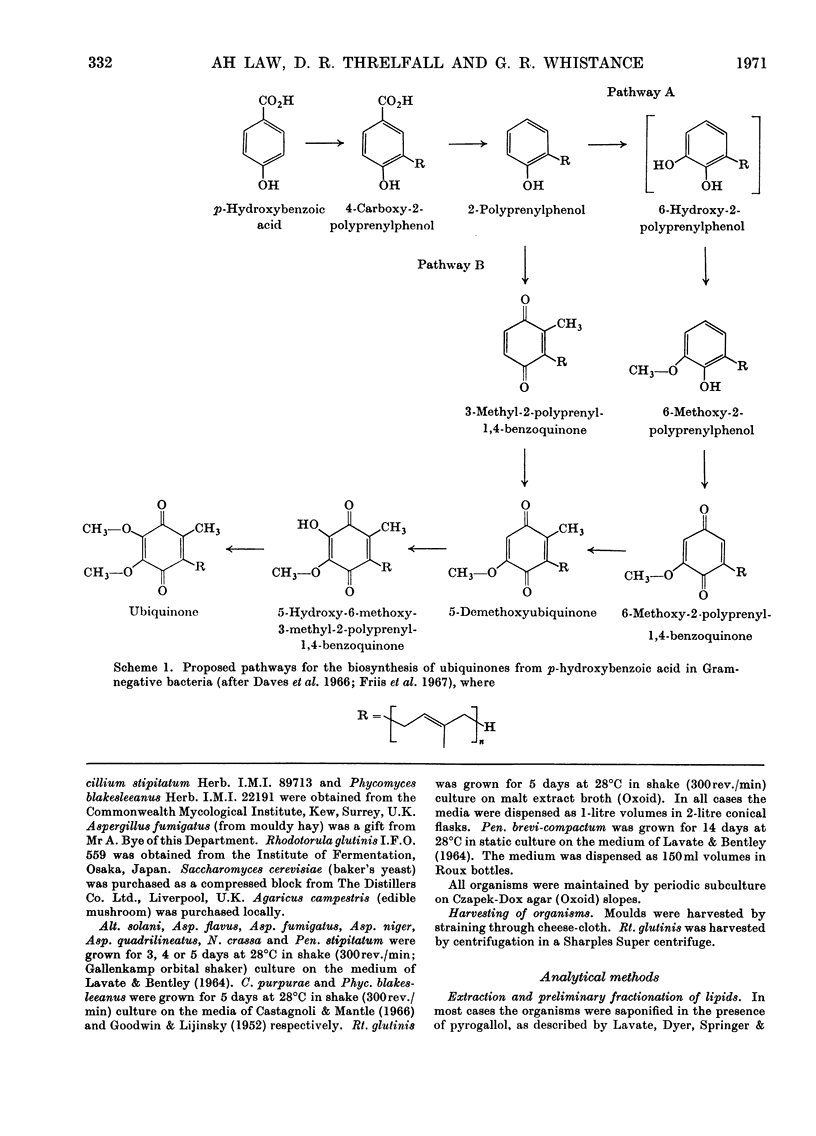
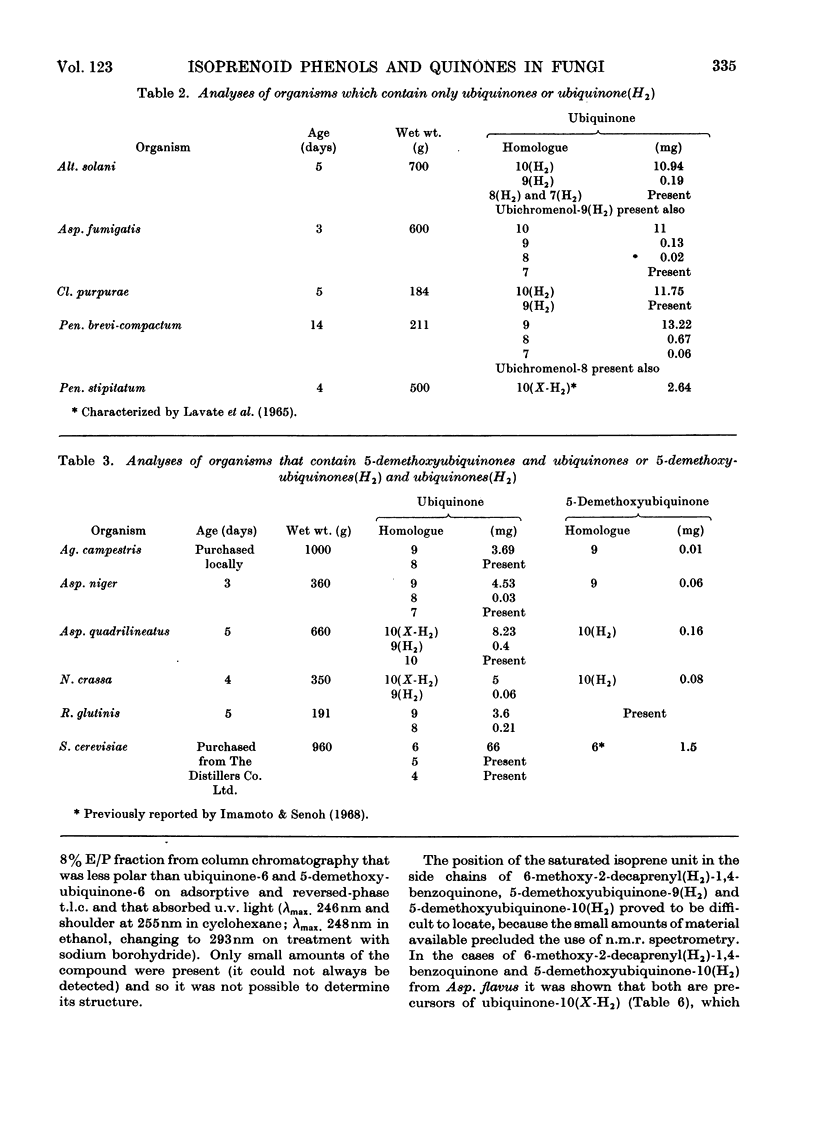
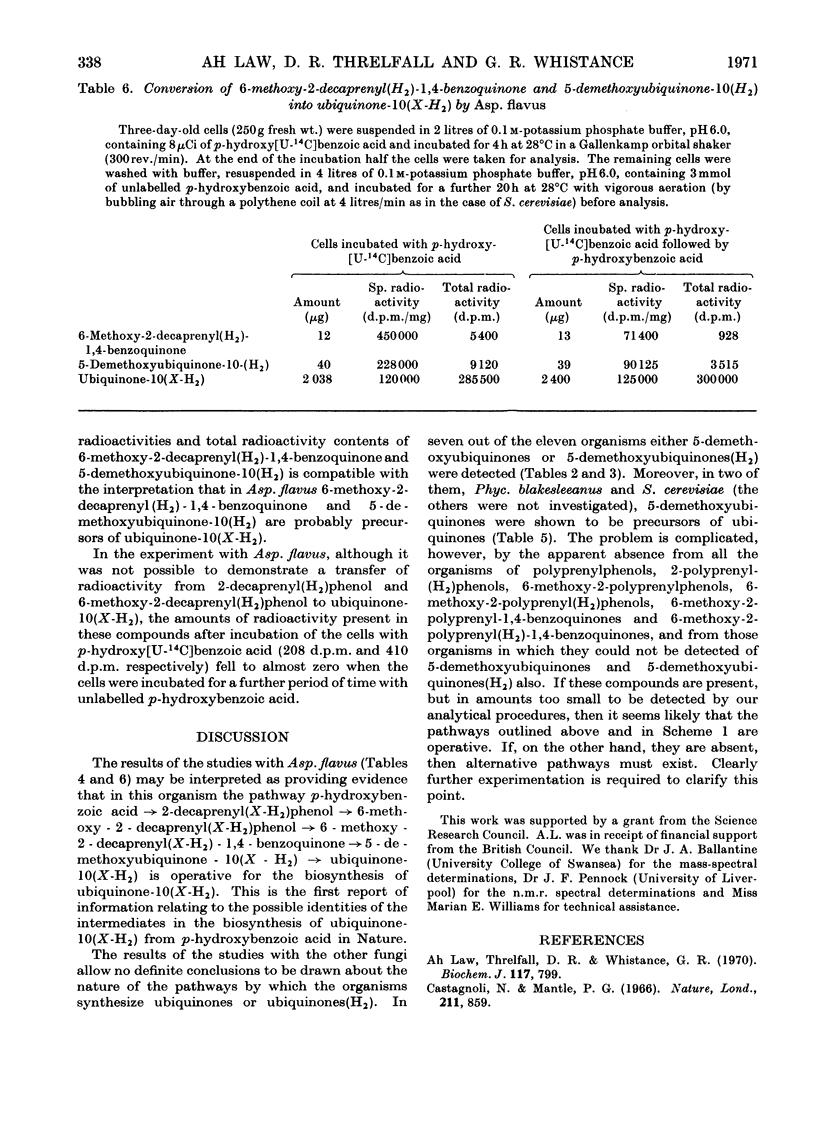
1. Ten moulds and two yeasts were analysed for the presence of 2-polyprenylphenols, 2-polyprenyl(H2)phenols, 6-methoxy-2-polyprenylphenols, 6-methoxy-2-polyprenyl(H2)phenols, 6-methoxy-2-polyprenyl-1,4-benzoquinones, 6-methoxy-2-polyprenyl(H2)-1,4-benzoquinones, 5-demethoxyubiquinones, 5-demethoxyubiquinones(H2), ubiquinones and ubiquinones(H2). 2. The organisms were found to be of three types: (a) those that contained only ubiquinones (Aspergillus fumigatus and Penicillium brevi-compactum) or ubiquinones(H2) (Alternaria solani, Claviceps purpurae and Penicillium stipitatum); (b) those that contained 5-demethoxyubiquinones and ubiquinones (Agaricus campestris, Aspergillus niger, Phycomyces blakesleeanus, Rhodotorula glutinis and Saccharomyces cerevisiae) or 5-demethoxyubiquinones(H2) and ubiquinones(H2) (Aspergillus quadrilineatus and Neurospora crassa); (c) one that contained 2-decaprenyl(H2)phenol, 6-methoxy-2-decaprenyl(H2)phenol, 6-methoxy-2-decaprenyl(X-H2)-1,4-benzoquinone, 5-demethoxyubiquinone-10(X-H2) and ubiquinones(H2) (Aspergillus flavus). 3. Studies were made on the biosynthesis of ubiquinones and ubiquinones(H2) by Asp. flavus, Phyc. blakesleeanus and S. cerevisiae. These provided evidence that in Phyc. blakesleeanus 5-demethoxyubiquinone-9 is a precursor of ubiquinone-9 and that in S. cerevisiae 5-demethoxyubiquinone-6 is a precursor of ubiquinone-6. In addition they yielded results that may be interpreted as providing evidence that in Asp. flavus 6-methoxy-2-decaprenyl(X-H2)-1,4-benzoquinone and 5-demethoxyubiquinone-10(X-H2) are precursors of ubiquinone-10(X-H2).
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castagnoli N., Jr, Mantle P. G. Occurrence of D-lysergic acid and 6-methylergol-8-ene-8-carboxylic acid in cultures of Claviceps purpurea. Nature. 1966 Aug 20;211(5051):859–860. doi: 10.1038/211859b0. [DOI] [PubMed] [Google Scholar]
- Daves G. D., Jr, Friis P., Olsen R. K., Folkers K. The chemistry of ubiquinone. Vitam Horm. 1966;24:427–439. doi: 10.1016/s0083-6729(08)60214-9. [DOI] [PubMed] [Google Scholar]
- Daves G. D., Jr, Wilczynski J. J., Friis P., Folkers K. Synthesis of rhodoquinone and other multiprenyl-1,4-benzoquinones biosynthetically related to ubiquinone. J Am Chem Soc. 1968 Sep 25;90(20):5587–5593. doi: 10.1021/ja01022a050. [DOI] [PubMed] [Google Scholar]
- Friis P., Nilsson J. L., Daves G. D., Jr, Folkers K. New multiprenylquinones in the biosynthesis of ubiquinone. Biochem Biophys Res Commun. 1967 Aug 7;28(3):324–327. doi: 10.1016/0006-291x(67)90312-9. [DOI] [PubMed] [Google Scholar]
- GALE P. H., ARISON B. H., TRENNER N. R., PAGE AC Jr FOLKERS K. Coenzyme Q. 36. Isolation and characterization of coenzyme Q10 (H-10). Biochemistry. 1963 Jan-Feb;2:196–200. doi: 10.1021/bi00901a037. [DOI] [PubMed] [Google Scholar]
- GOODWIN T. W., LIJINSKY W. Studies in carotenogenesis. II. Carotene production by Phycomyces blakesleeanus; the effect of different amino-acids when used in media containing low concentrations of glucose. Biochem J. 1951 Dec;50(2):268–273. doi: 10.1042/bj0500268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAVATE W. V., BENTLEY R. DISTRIBUTION OF NORMAL ISOPRENOLOGS OF COENZYME Q AND DIHYDRO COENZYME Q10 IN VARIOUS MOLDS. Arch Biochem Biophys. 1964 Nov;108:287–291. doi: 10.1016/0003-9861(64)90389-3. [DOI] [PubMed] [Google Scholar]
- LAVATE W. V., DYER J. R., SPRINGER C. M., BENTLEY R. STUDIES ON COENZYME Q. THE ISOLATION, CHARACTERIZATION, AND GENERAL PROPERTIES OF A PARTLY REDUCED COENZYME Q10 FROM PENICILLIUM STIPITATUM. J Biol Chem. 1965 Jan;240:524–531. [PubMed] [Google Scholar]
- LAWSON D. E., THRELFALL D. R., GLOVER J., MORTON R. A. Biosynthesis of ubiquinone in the rat. Biochem J. 1961 Apr;79:201–208. doi: 10.1042/bj0790201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER R. L., CRANE F. L. The natural occurrence of coenzyme Q and related compounds. J Biol Chem. 1959 Aug;234(8):2169–2175. [PubMed] [Google Scholar]
- Law A., Threlfall D. R., Whistance G. R. Isoprenoid quinone precursors of ubiquinone-10(X-H2) in Aspergillus flavus. Biochem J. 1970 May;117(4):799–800. doi: 10.1042/bj1170799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. K., Daves G. D., Jr, Moore H. W., Folkers K., Parson W. W., Rudney H. 2-multiprenylphenols and 2-decaprenyl-6-methoxyphenol, biosynthetic precursors of ubiquinones. J Am Chem Soc. 1966 Dec 20;88(24):5919–5923. doi: 10.1021/ja00976a036. [DOI] [PubMed] [Google Scholar]
- PACKTER N. M., GLOVER J. Ubiquinone (50) and ubichromenol in Aspergillus fumigatus Fresenius. Nature. 1960 Jul 30;187:413–414. doi: 10.1038/187413b0. [DOI] [PubMed] [Google Scholar]
- PARSON W. W., RUDNEY H. AN INTERMEDIATE IN THE CONVERSION OF P-HYDROXYBENZOATE-U-C-14 TO UBIQUINONE IN RHODOSPIRILLUM RUBRUM. Proc Natl Acad Sci U S A. 1965 Mar;53:599–606. doi: 10.1073/pnas.53.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARSON W. W., RUDNEY H. THE BIOSYNTHESIS OF THE BENZOQUINONE RING OF UBIQUINONE FROM P-HYDROXYBENZALDEHYDE AND P-HYDROXYBENZOIC ACID IN RAT KIDNEY, AZOTOBACTER VINELANDII, AND BAKER'S YEAST. Proc Natl Acad Sci U S A. 1964 Mar;51:444–450. doi: 10.1073/pnas.51.3.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller G. H., Threlfall D. R., Whistance G. R. Biosynthesis of ubiquinone in yeast, Phycomyces blakesleeanus, and Agaricus campestris. Arch Biochem Biophys. 1968 Jun;125(3):786–796. doi: 10.1016/0003-9861(68)90515-8. [DOI] [PubMed] [Google Scholar]
- Threlfall D. R., Goodwin T. W. Nature, intracellular distribution and formation of terpenoid quinones in Euglena gracilis. Biochem J. 1967 May;103(2):573–588. doi: 10.1042/bj1030573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfall D. R., Whistance G. R., Goodwin T. W. Biosynthesis of phytoquinones. Incorporation of L-[Me-14C,3H]methionine into terpenoid quinones and chromanols in maize shoots. Biochem J. 1968 Jan;106(1):107–112. doi: 10.1042/bj1060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistance G. R., Brown B. S., Threlfall D. R. Biosynthesis of ubiquinone in non-photosynthetic gram-negative bacteria. Biochem J. 1970 Mar;117(1):119–128. doi: 10.1042/bj1170119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistance G. R., Brown B. S., Threlfall D. R. Isolation of possible ubiquinone precursors from nonphotosynthetic Gram-negative bacteria. Biochim Biophys Acta. 1969 Jun 10;176(4):895–897. doi: 10.1016/0005-2760(69)90275-6. [DOI] [PubMed] [Google Scholar]
- Whistance G. R., Dillon J. F., Threlfall D. R. The nature, intergeneric distribution and biosynthesis of isoprenoid quinones and phenols in gram-negative bacteria. Biochem J. 1969 Feb;111(4):461–472. doi: 10.1042/bj1110461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistance G. R., Threlfall D. R., Goodwin T. W. Observations on the biosynthesis of phytoterpenoid quinone and chromanol nuclei. Biochem J. 1967 Oct;105(1):145–154. doi: 10.1042/bj1050145. [DOI] [PMC free article] [PubMed] [Google Scholar]


