Abstract
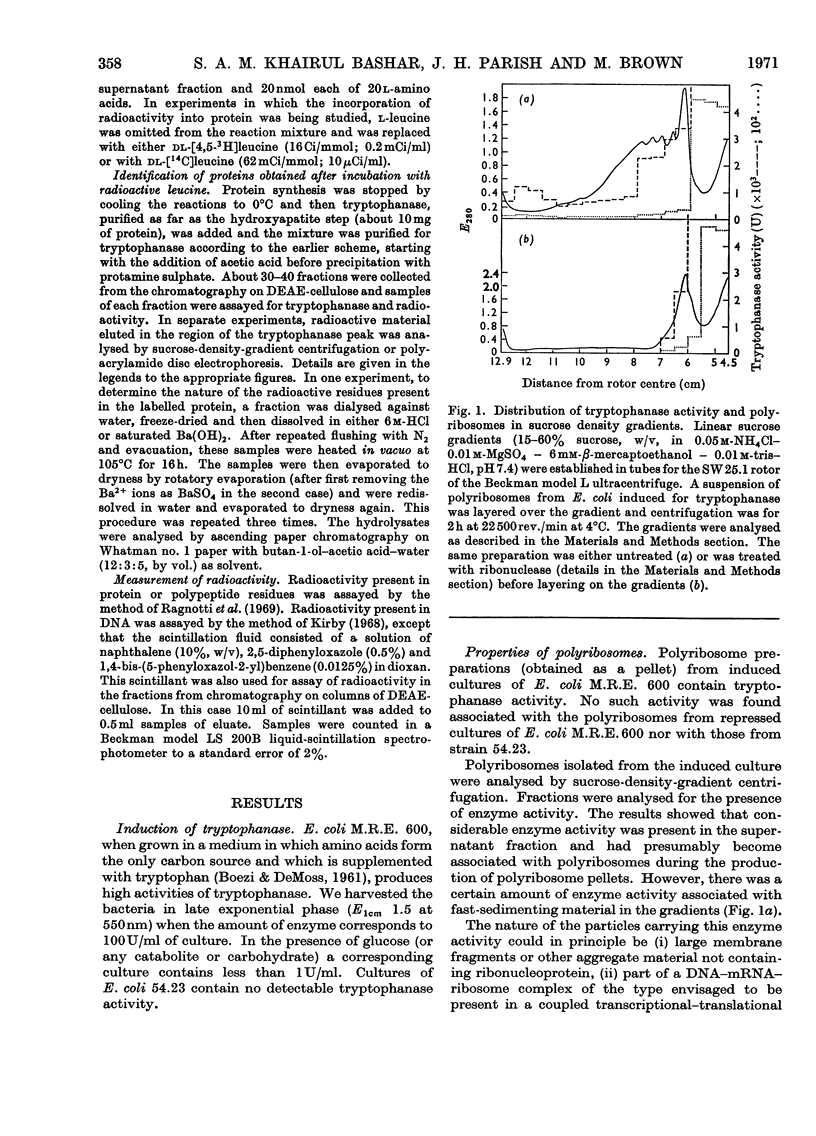
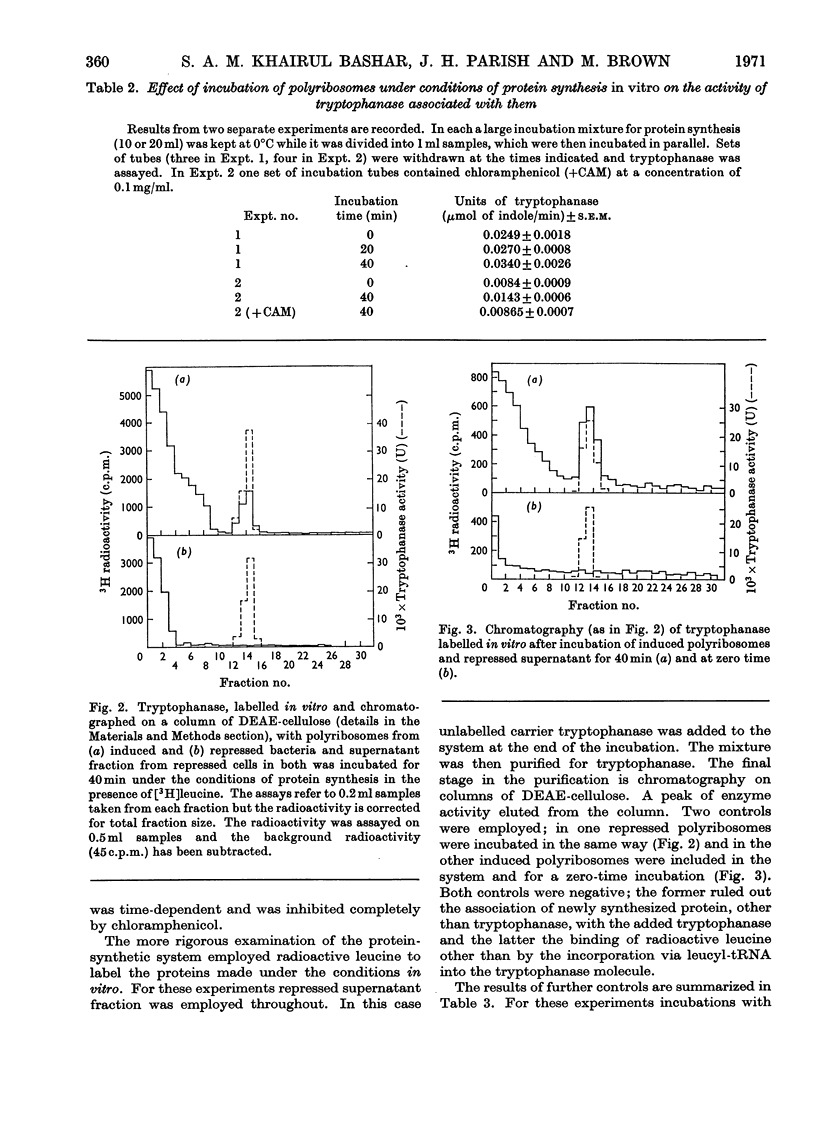
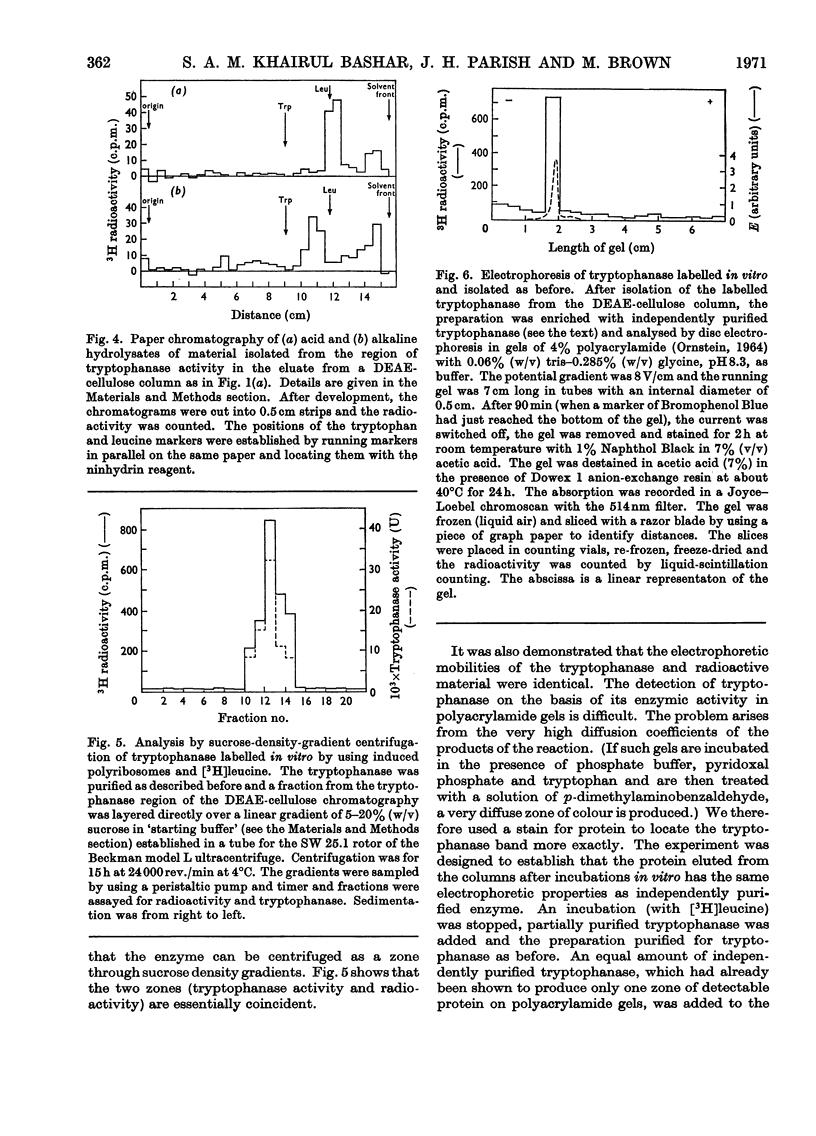
1. Polyribosomes were isolated from Escherichia coli grown in media in which tryptophanase is induced and in which it is repressed. The polyribosomes from the induced bacteria had a small amount of tryptophanase activity associated with them. 2. A portion of the enzyme activity remained bound to polyribosomes during centrifuging in sucrose gradients. 3. Incubation of tryptophanase-containing polyribosomes with puromycin released enzyme activity. 4. The binding of the enzyme to the polyribosomes did not depend on the presence of DNA. 5. When the polyribosomes were incubated under conditions of protein synthesis with supernatant fraction obtained from repressed bacteria, a small but statistically significant increase in enzyme activity was produced. 6. When a radioactive amino acid was included in the incubation mixture for the tryptophanase system a radioactive protein was obtained whose chromatographic, electrophoretic and sedimentation properties were identical with those of tryptophanase. 7. The amount of incorporation was consistent with the amount of new enzyme synthesis predicted by the increase in enzyme activity. Both radioactive incorporation and increase in enzyme activity were shown to be energy-dependent and also negative controls were obtained by using zero-time incubations or polyribosomes isolated from either repressed cells or a mutant lacking the ability to produce tryptophanase. 8. The distribution of radioactive leucine in the carboxyl region of the newly labelled tryptophanase was examined by digesting the labelled protein with carboxypeptidases. It was shown that the radioactivity was more highly concentrated towards the carboxyl terminus when the incubation times for protein synthesis were shorter (implying that, with longer incubation times, longer lengths of polypeptide chain contained radioactive amino acid residues).
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNS R. O., DEMOSS R. D. Properties of tryptophanase from Escherichia coli. Biochim Biophys Acta. 1962 Dec 4;65:233–244. doi: 10.1016/0006-3002(62)91042-9. [DOI] [PubMed] [Google Scholar]
- Campbell P. N. Functions of polyribosomes attached to membranes of animal cells. FEBS Lett. 1970 Mar 16;7(1):1–7. doi: 10.1016/0014-5793(70)80603-2. [DOI] [PubMed] [Google Scholar]
- Cannon M. The puromycin reaction and its inhibition by chloramphenicol. Eur J Biochem. 1968 Dec;7(1):137–145. doi: 10.1111/j.1432-1033.1968.tb19584.x. [DOI] [PubMed] [Google Scholar]
- HJERTEN S., LEVIN O., TISELIUS A. Protein chromatography on calcium phosphate columns. Arch Biochem Biophys. 1956 Nov;65(1):132–155. doi: 10.1016/0003-9861(56)90183-7. [DOI] [PubMed] [Google Scholar]
- KIHO Y., RICH A. INDUCED ENZYME FORMED ON BACTERIAL POLYRIBOSOMES. Proc Natl Acad Sci U S A. 1964 Jan;51:111–118. doi: 10.1073/pnas.51.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiho Y., Rich A. A polycistronic messenger RNA associated with beta-galactosidase induction. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1751–1758. doi: 10.1073/pnas.54.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morino Y., Snell E. E. The subunit structure of tryptophanase. I. The effect of pyridoxal phosphate on the subunit structure and physical properties of tryptophanase. J Biol Chem. 1967 Dec 10;242(23):5591–5601. [PubMed] [Google Scholar]
- Morino Y., Snell E. E. The subunit structure of tryptophanase. II. A correlation of ultracentrifugal and chemical studies. J Biol Chem. 1967 Dec 10;242(23):5602–5610. [PubMed] [Google Scholar]
- NEWTON W. A., SNELL E. E. An inducible tryptophan synthetase in tryptophan auxotrophs of Escherichia coli. Proc Natl Acad Sci U S A. 1962 Aug;48:1431–1439. doi: 10.1073/pnas.48.8.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON W. A., SNELL E. E. CATALYTIC PROPERTIES OF TRYPTOPHANASE, A MULTIFUNCTIONAL PYRIDOXAL PHOSPHATE ENZYME. Proc Natl Acad Sci U S A. 1964 Mar;51:382–389. doi: 10.1073/pnas.51.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- PITTARD J. EFFECT OF INTEGRATED SEX FACTOR ON TRANSDUCTION OF CHROMOSOMAL GENES IN ESCHERICHIA COLI. J Bacteriol. 1965 Mar;89:680–686. doi: 10.1128/jb.89.3.680-686.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish J. H. Fractions of RNA and ribonucleoprotein from bacterial polysomes. I. Isolation of polysomes from Escherichia coli. Biochim Biophys Acta. 1969 Jun 17;182(2):454–460. doi: 10.1016/0005-2787(69)90197-x. [DOI] [PubMed] [Google Scholar]
- Ragnotti G., Lawford G. R., Campbell P. N. Biosynthesis of microsomal nicotinamide-adenine dinucleotide phosphate-cytochrome c reductase by membrane-bound and free polysomes from rat liver. Biochem J. 1969 Apr;112(2):139–147. doi: 10.1042/bj1120139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecke C. J., van Reisen R., Voorma H. O., Bosch L. Chain initiation during polypeptide synthesis in a cell-free bacterial system programmed with plant viral messengers. I. Dependence on formylation and ribosomal factors. Biochim Biophys Acta. 1968 May 21;157(3):566–578. doi: 10.1016/0005-2787(68)90154-8. [DOI] [PubMed] [Google Scholar]
- Stahl J., Lawford G. R., Williams B., Campbell P. N. A requirement for the presence of cell sap in the reversible dissociation of rat liver polysomes to ribosomal sub-units. Biochem J. 1968 Aug;109(1):155–157. doi: 10.1042/bj1090155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Stephenson M. L., Scott J. F. PARTIAL PURIFICATION OF SOLUBLE RNA. Proc Natl Acad Sci U S A. 1960 Jun;46(6):811–822. doi: 10.1073/pnas.46.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G., Chambers D. A. A DNA-directed cell-free system for beta-galactosidase synthesis; characterization of the de novo synthesized enzyme and some aspects of the regulation of synthesis. Cold Spring Harb Symp Quant Biol. 1969;34:753–761. doi: 10.1101/sqb.1969.034.01.085. [DOI] [PubMed] [Google Scholar]


