Abstract
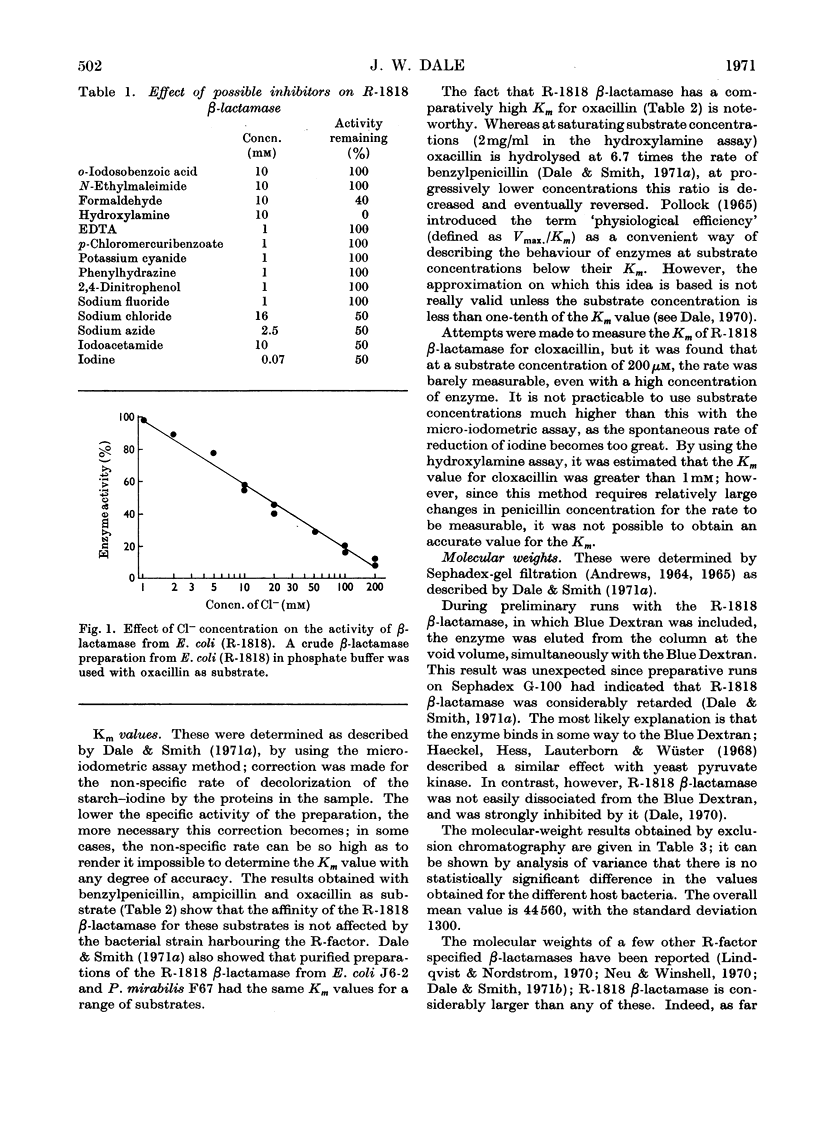
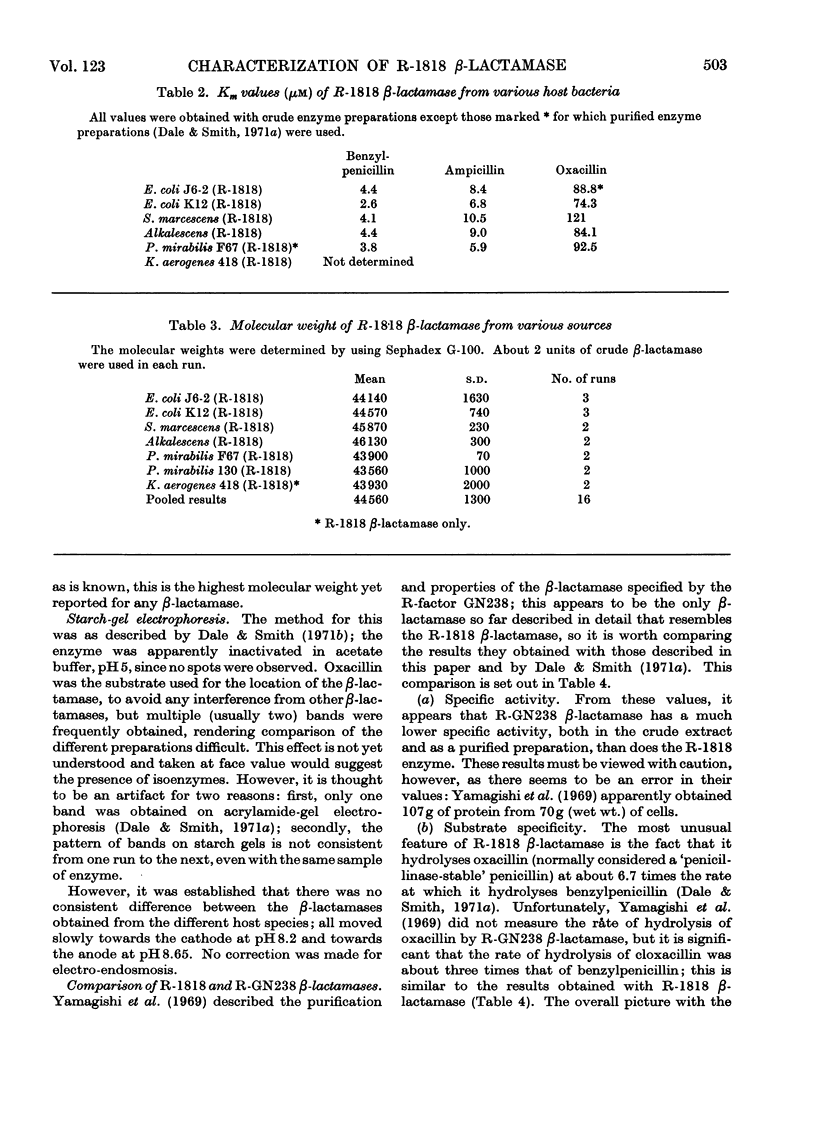
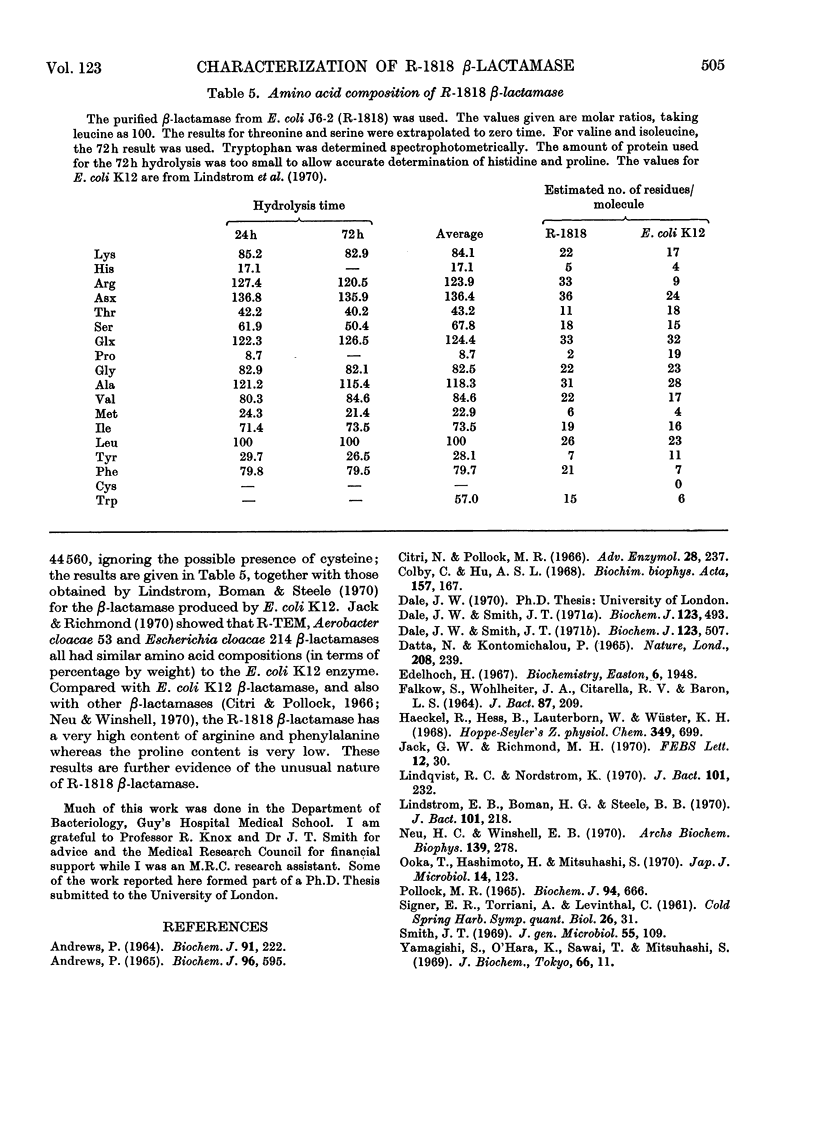
1. The amino acid composition of the β-lactamase from E. coli (R-1818) was determined. 2. The R-1818 β-lactamase is inhibited by formaldehyde, hydroxylamine, sodium azide, iodoacetamide, iodine and sodium chloride. 3. The Km values for benzylpenicillin, ampicillin and oxacillin have been determined by using the R-factor enzyme from different host species. The same values were obtained, irrespective of the host bacterium. 4. The molecular weight of the enzyme was found to be 44600, and was the same for all host species. 5. The relationship of R-1818 and R-GN238 β-lactamases is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri N., Pollock M. R. The biochemistry and function of beta-lactamase (penicillinase). Adv Enzymol Relat Areas Mol Biol. 1966;28:237–323. doi: 10.1002/9780470122730.ch4. [DOI] [PubMed] [Google Scholar]
- Colby C., Jr, Hu A. S. Purification and comparison of the beta-galactosidase synthesized by Escherichia coli F-lac and Proteus mirabilis F-lac. Biochim Biophys Acta. 1968 Mar 18;157(1):167–177. doi: 10.1016/0005-2787(68)90275-x. [DOI] [PubMed] [Google Scholar]
- Dale J. W., Smith J. T. Some relationships between R-factor and chromosomal -lactamase in Gram-negative bacteria. Biochem J. 1971 Jul;123(4):507–512. doi: 10.1042/bj1230507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. W., Smith J. T. The purification and properties of the -lactamase specified by the resistance factor R-1818 in Escherichia coli and Proteus mirabilis. Biochem J. 1971 Jul;123(4):493–500. doi: 10.1042/bj1230493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Kontomichalou P. Penicillinase synthesis controlled by infectious R factors in Enterobacteriaceae. Nature. 1965 Oct 16;208(5007):239–241. doi: 10.1038/208239a0. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- FALKOW S., WOHLHIETER J. A., CITARELLA R. V., BARON L. S. TRANSFER OF EPISOMIC ELEMENTS TO PROTEUS. I. TRANSFER OF F-LINKED CHROMOSOMAL DETERMINANTS. J Bacteriol. 1964 Jan;87:209–219. doi: 10.1128/jb.87.1.209-219.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeckel R., Hess B., Lauterborn W., Wüster K. H. Purification and allosteric properties of yeast pyruvate kinase. Hoppe Seylers Z Physiol Chem. 1968 May;349(5):699–714. doi: 10.1515/bchm2.1968.349.1.699. [DOI] [PubMed] [Google Scholar]
- Jack G. W., Richmond M. H. Comparative amino acid contents of purified beta-lactamases from enteric bacteria. FEBS Lett. 1970 Dec 23;12(1):30–32. doi: 10.1016/0014-5793(70)80587-7. [DOI] [PubMed] [Google Scholar]
- Lindqvist R. C., Nordström K. Resistance of Escherichia coli to penicillins. VII. Purification and characterization of a penicillinase mediated by the R factor R1. J Bacteriol. 1970 Jan;101(1):232–239. doi: 10.1128/jb.101.1.232-239.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linström E. B., Boman H. G., Steele B. B. Resistance of Escherichia coli to penicillins. VI. Purification and characterization of the chromosomally mediated penicillinase present in ampA-containing strains. J Bacteriol. 1970 Jan;101(1):218–231. doi: 10.1128/jb.101.1.218-231.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Winshell E. B. Purification and characterization of penicillinases from Salmonella typhimurium and Escherichia coli. Arch Biochem Biophys. 1970 Aug;139(2):278–290. doi: 10.1016/0003-9861(70)90479-0. [DOI] [PubMed] [Google Scholar]
- Ooka T., Hashimoto H., Mitsuhashi S. Comparison of penicillinases produced by R factors isolated from ampicillin-resistant gram-negative bacteria. Jpn J Microbiol. 1970 Mar;14(2):123–128. doi: 10.1111/j.1348-0421.1970.tb00499.x. [DOI] [PubMed] [Google Scholar]
- POLLOCK M. R. PURIFICATION AND PROPERTIES OF PENICILLINASES FROM TWO STRAINS OF BACILLUS LICHENIFORMIS: A CHEMICAL, PHYSICOCHEMICAL AND PHYSIOLOGICAL COMPARISON. Biochem J. 1965 Mar;94:666–675. doi: 10.1042/bj0940666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIGNER E. R., TORRIANI A., LEVINTHAL C. Gene expression in intergeneric merozygotes. Cold Spring Harb Symp Quant Biol. 1961;26:31–34. doi: 10.1101/sqb.1961.026.01.008. [DOI] [PubMed] [Google Scholar]
- Smith J. T. R-factor gene expression gram-negative bacteria. J Gen Microbiol. 1969 Jan;55(1):109–120. doi: 10.1099/00221287-55-1-109. [DOI] [PubMed] [Google Scholar]


