Abstract
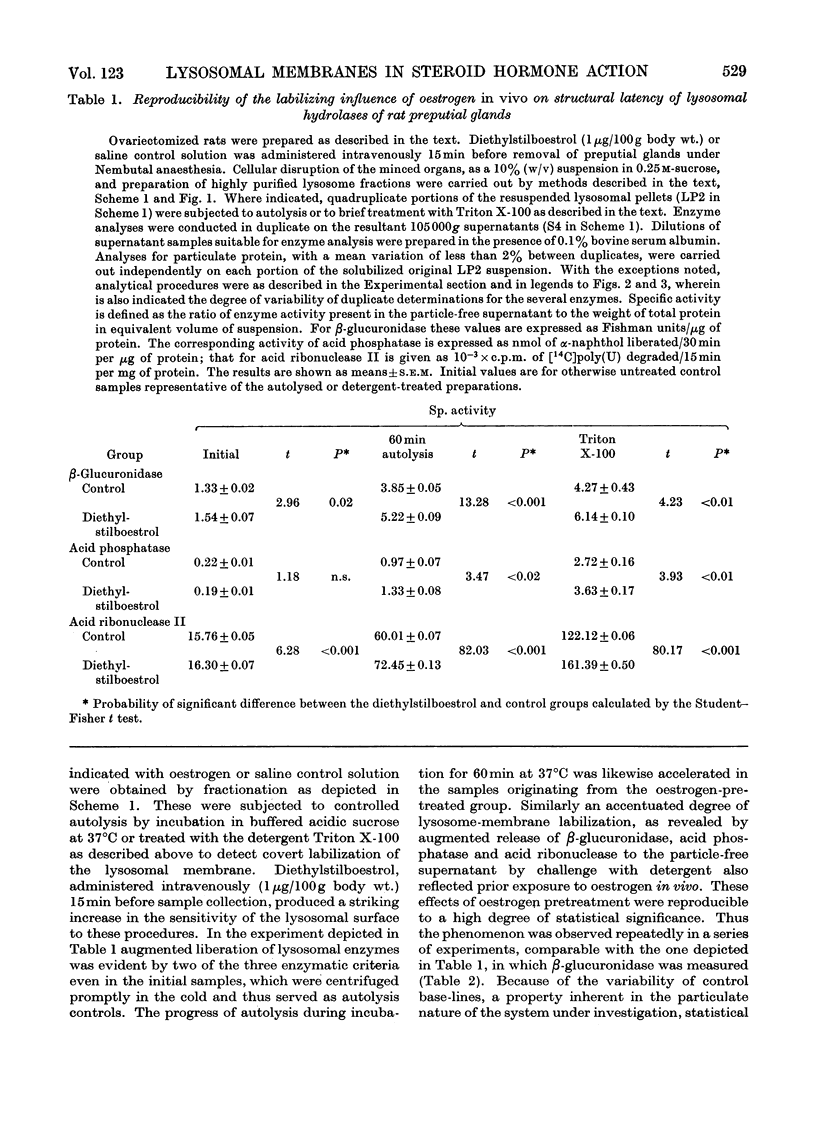
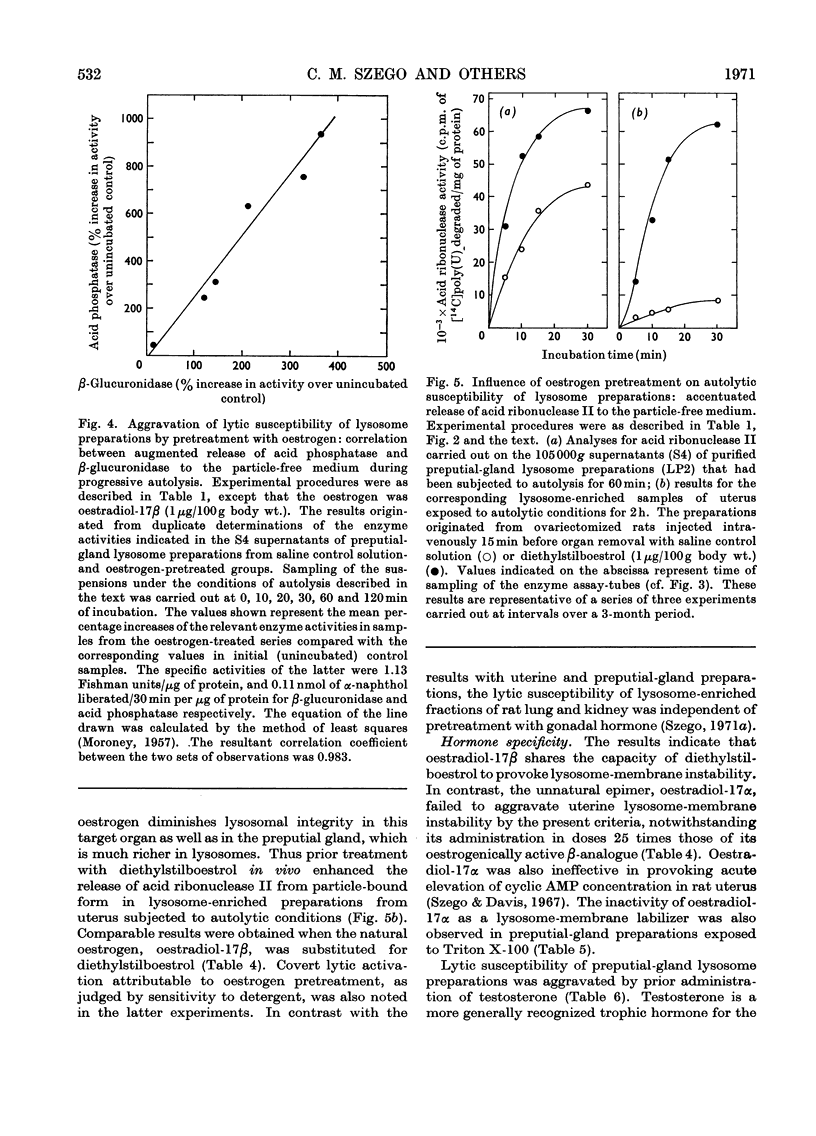
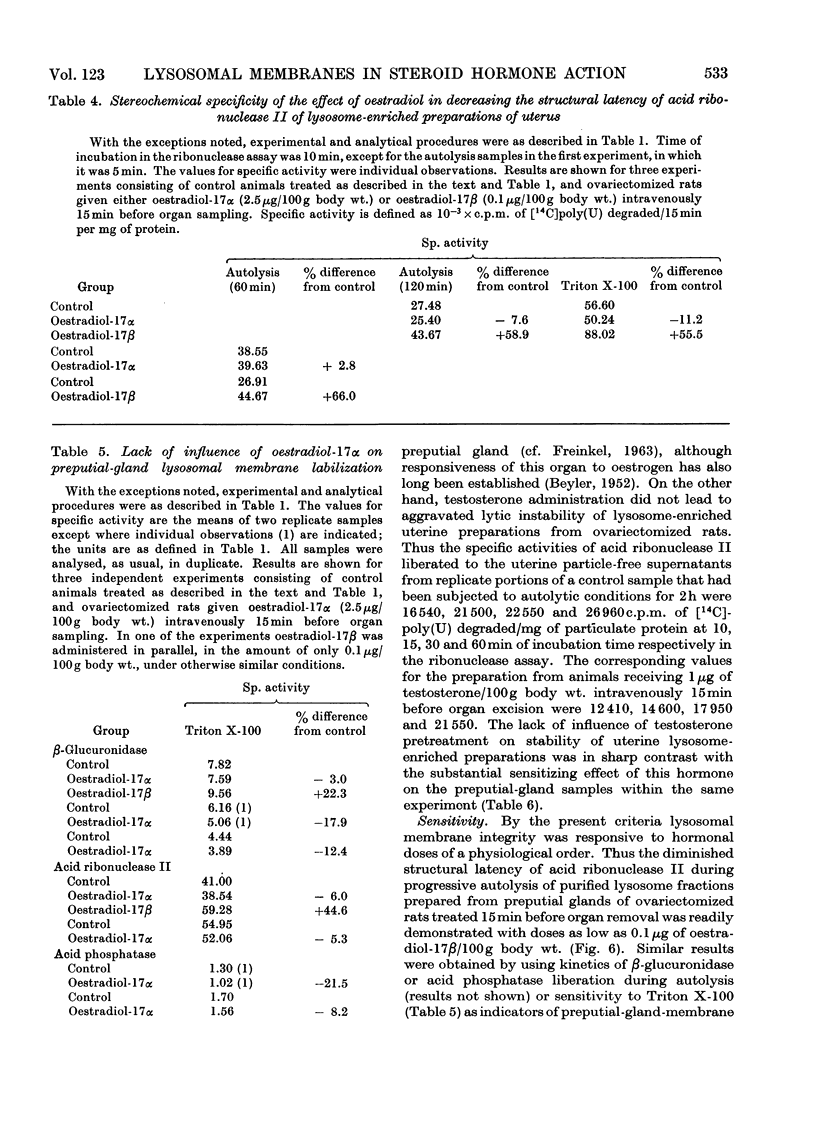
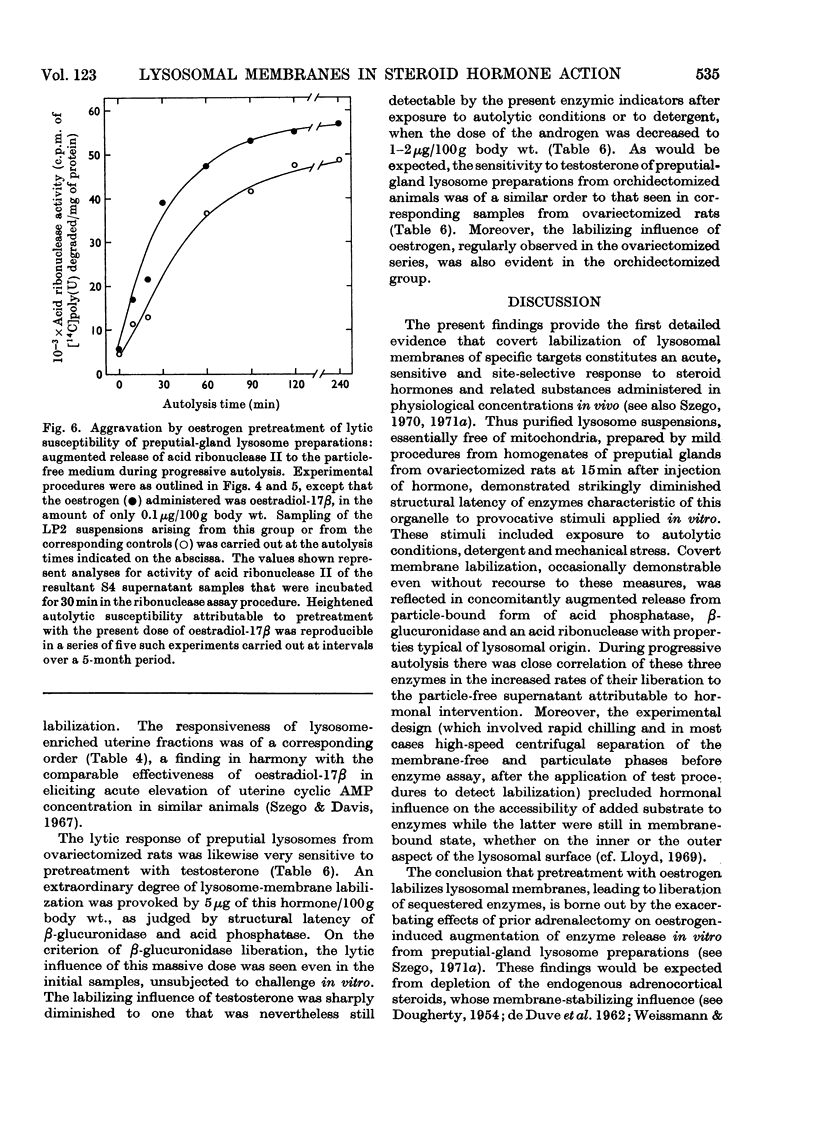
At short intervals after the intravenous administration of oestradiol-17β, diethylstilboestrol, testosterone or saline control solution to ovariectomized rats, highly purified lysosome samples were prepared in substantial yield from preputial glands, sex accessory organs rich in these organelles. The preparations were essentially devoid of mitochondrial contamination. Exposure in vivo to doses of these hormones varying from 0.1 to 5μg/100g body wt. provoked dose-dependent labilization of the lysosomal membrane surface, as evidenced by significantly diminished structural latency of several characteristic acid hydrolases, including acid phosphatase, β-glucuronidase and acid ribonuclease II, when such preparations were subsequently challenged in vitro with autolytic conditions, detergent or mechanical stress. Enhanced lytic susceptibility induced by hormone pretreatment was occasionally detectable in the initial preparation without further provocative stimuli in vitro. Comparable results were obtained with the corresponding fractions of uterus, despite the more limited concentration of lysosomes in this steroidal target organ. By the present criteria oestradiol-17α was essentially inert, even in a dose 25 times that effective for its active β-epimer (<0.1μg/100g body wt.). Pretreatment with diethylstilboestrol exerted substantial membrane-destabilizing influence in preputial-gland lysosome samples from orchidectomized rats. Moreover, administration of testosterone to gonadectomized animals resulted in essentially equivalent dose-dependent augmentation of lysosomal enzyme release in preputial-gland preparations of either sex. The membrane stability of lysosome-enriched preparations from uterus, on the other hand, was unaffected by testosterone pretreatment. The sensitivity, specificity and selectivity of the lysosomal response to sex steroids provide evidence for the physiological significance of this phenomenon as a general mechanism for mediation of secondary biochemical transformations in the hormone-stimulated target cell.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., MALLUCCI L. LYSOSOMES IN DIVIDING CELLS, WITH SPECIAL REFERENCE TO LYMPHOCYTES. Lancet. 1964 Dec 26;2(7374):1371–1373. doi: 10.1016/s0140-6736(64)91162-6. [DOI] [PubMed] [Google Scholar]
- APPELMANS F., WATTIAUX R., DE DUVE C. Tissue fractionation studies. 5. The association of acid phosphatase with a special class of cytoplasmic granules in rat liver. Biochem J. 1955 Mar;59(3):438–445. doi: 10.1042/bj0590438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison A. The role of lysosomes in the action of drugs and hormones. Adv Chemother. 1968;3:253–302. [PubMed] [Google Scholar]
- BEYLER A. L., SZEGO C. M. Steroid hormone interactions as evidenced by modification of beta-glucuronidase activity of the preputial glands of the rat. Endocrinology. 1954 Mar;54(3):334–337. doi: 10.1210/endo-54-3-334. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard E. A. Ribonucleases. Annu Rev Biochem. 1969;38:677–732. doi: 10.1146/annurev.bi.38.070169.003333. [DOI] [PubMed] [Google Scholar]
- Barondes S. H., Nirenberg M. W. Fate of a Synthetic Polynucleotide Directing Cell-Free Protein Synthesis I. Characteristics of Degradation. Science. 1962 Nov 16;138(3542):810–813. doi: 10.1126/science.138.3542.810. [DOI] [PubMed] [Google Scholar]
- Billing R. J., Barbiroli B., Smellie R. M. The mode of action of oestradiol. I. The transport of RNA precursors into the uterus. Biochim Biophys Acta. 1969 Sep 17;190(1):52–59. [PubMed] [Google Scholar]
- Billing R. J., Barbiroli B., Smellie R. M. The mode of action of oestradiol. II. The synthesis of RNA. Biochim Biophys Acta. 1969 Sep 17;190(1):60–65. doi: 10.1016/0005-2787(69)90154-3. [DOI] [PubMed] [Google Scholar]
- CAMPBELL D. M., MOSS D. W. Spectrofluorimetric determination of acid phosphatase activity. Clin Chim Acta. 1961 May;6:307–315. doi: 10.1016/0009-8981(61)90055-9. [DOI] [PubMed] [Google Scholar]
- Catchpole H. R. Effects of hormones on connective tissue. Fed Proc. 1966 May-Jun;25(3):1144–1145. [PubMed] [Google Scholar]
- DE DUVE C., WATTIAUX R., WIBO M. Effects of fat-soluble compounds on lysosomes in vitro. Biochem Pharmacol. 1962 Aug;9:97–116. doi: 10.1016/0006-2952(62)90015-1. [DOI] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Duncan C. J. Properties and stabilization of the lysosomal membrane. Nature. 1966 Jun 18;210(5042):1229–1230. doi: 10.1038/2101229a0. [DOI] [PubMed] [Google Scholar]
- EMANUEL C. F., CHAIKOFF I. L. An hydraulic homogenizer for the controlled release of cellular components from various tissues. Biochim Biophys Acta. 1957 May;24(2):254–261. doi: 10.1016/0006-3002(57)90191-9. [DOI] [PubMed] [Google Scholar]
- Fell H. B. Role of biological membranes in some skeletal reactions. Ann Rheum Dis. 1969 May;28(3):213–227. doi: 10.1136/ard.28.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman W. H., Goldman S. S., DeLellis R. Dual localization of beta-glucuronidase in endoplasmic reticulum and in lysosomes. Nature. 1967 Feb 4;213(5075):457–460. doi: 10.1038/213457a0. [DOI] [PubMed] [Google Scholar]
- Griffin D. M., Szego C. M. Adenosine 3',5'-monophosphate stimulation of uterine amino acid uptake in vitro. Life Sci. 1968 Oct 15;7(20):1017–1023. doi: 10.1016/0024-3205(68)90137-9. [DOI] [PubMed] [Google Scholar]
- HECHTER O. Concerning possible mechanisms of hormone action. Vitam Horm. 1955;(13):293–346. doi: 10.1016/s0083-6729(08)61029-8. [DOI] [PubMed] [Google Scholar]
- Hirsch H. E. Acid phosphatase localization in individual neurons by a quantitative histochemical method. J Neurochem. 1968 Feb;15(2):123–130. doi: 10.1111/j.1471-4159.1968.tb06183.x. [DOI] [PubMed] [Google Scholar]
- KANDUTSCH A. A., RUSSELL A. E. Preputial gland tumor sterols. 2. The identification of 4 alpha-methyl-Delta 8-cholesten-3 beta-ol. J Biol Chem. 1960 Aug;235:2253–2255. [PubMed] [Google Scholar]
- LEVVY G. A., McALLAN A., MARSH C. A. Purification of beta-glucuronidase from the preputial gland of the female rat. Biochem J. 1958 May;69(1):22–27. doi: 10.1042/bj0690022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lloyd J. B. Studies on the permeability of rat liver lysosomes to carbohydrates. Biochem J. 1969 Dec;115(4):703–707. doi: 10.1042/bj1150703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUSA B. U., DOE R. P., SEAL U. S. PURIFICATION AND PROPERTIES OF HUMAN LIVER BETA-GLUCURONIDASE. J Biol Chem. 1965 Jul;240:2811–2816. [PubMed] [Google Scholar]
- ROBERTS S., SZEGO C. M. Steroid interaction in the metabolism of reproductive target organs. Physiol Rev. 1953 Oct;33(4):593–629. doi: 10.1152/physrev.1953.33.4.593. [DOI] [PubMed] [Google Scholar]
- Razzell W. E. Polynucleotidases in animal tissues. Experientia. 1967 May 15;23(5):321–325. doi: 10.1007/BF02144490. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Fishman W. H. p-(acetoxymercuric) aniline diazotate, a reagent for visualizing the naphthol AS-BI product of acid hydrolase action at the level of the light and electron microscope. J Histochem Cytochem. 1969 Jan;17(1):1–22. doi: 10.1177/17.1.1. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Henzl M. R. Role of mucopolysaccharides and lysosomal hydrolases in endometrial regression following withdrawal of estradiol and chlormadinone acetate. I. Epithelium and stroma. Endocrinology. 1969 Jul;85(1):50–66. doi: 10.1210/endo-85-1-50. [DOI] [PubMed] [Google Scholar]
- Szego C. M., Davis J. S. Adenosine 3',5'-monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1711–1718. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szego C. M., Davis J. S. Inhibition of estrogen-induced cyclic AMP elevation in rat uterus. II: By glucocorticoids. Life Sci. 1969 Oct 1;8(19):1109–1116. doi: 10.1016/0024-3205(69)90164-7. [DOI] [PubMed] [Google Scholar]
- Szego C. M., Davis J. S. Inhibition of estrogen-induced elevation of cyclic 3',5'-adenosine monophosphate in rat uterus. I. By beta-adrenergic receptor-blocking drugs. Mol Pharmacol. 1969 Sep;5(5):470–480. [PubMed] [Google Scholar]
- Szego C. M. Role of histamine in mediation of hormone action. Fed Proc. 1965 Nov-Dec;24(6):1343–1352. [PubMed] [Google Scholar]
- Vignais P. M., Nachbaur J. A critical evaluation of the contamination, by lysosomes, of preparations of outer membrane of mitochondria. Biochem Biophys Res Commun. 1968 Oct 24;33(2):307–314. doi: 10.1016/0006-291x(68)90785-7. [DOI] [PubMed] [Google Scholar]
- WEISSMANN G., THOMAS L. Studies on lysosomes. II. The effect of cortisone on the release of acid hydrolases from a large granule fraction of rabbit liver induced by an excess of vitamin A. J Clin Invest. 1963 May;42:661–669. doi: 10.1172/JCI104757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSMANN G., THOMAS L. THE EFFECTS OF CORTICOSTEROIDS UPON CONNECTIVE TISSUE AND LYSOSOMES. Recent Prog Horm Res. 1964;20:215–245. [PubMed] [Google Scholar]
- Weissmann G., Hirschhorn R., Pras M., Sessa G., Bevans V. A. Studies of lysosomes. 8. The effect of polyene antibiotics on lysosomes. Biochem Pharmacol. 1967 Jun;16(6):1057–1069. doi: 10.1016/0006-2952(67)90279-1. [DOI] [PubMed] [Google Scholar]
- Weissmann G. Lysosome. N Engl J Med. 1965 Nov 11;273(20):1084–contd. doi: 10.1056/NEJM196511112732006. [DOI] [PubMed] [Google Scholar]
- Weissmann G. Lysosomes. N Engl J Med. 1965 Nov 18;273(21):1143–concl. doi: 10.1056/NEJM196511182732107. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr Acid hydrolases of the rat uterus in relation to pregnancy, post-partum involution and collagen breakdown. Biochem J. 1965 Dec;97(3):855–866. doi: 10.1042/bj0970855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonetani T. Studies on cytochrome c peroxidase. II. Stoichiometry between enzyme, H2O2, and ferrocytochrome c and enzymic determination of extinction coefficients of cytochrome c. J Biol Chem. 1965 Nov;240(11):4509–4514. [PubMed] [Google Scholar]
- Zomzely C. E., Roberts S., Gruber C. P., Brown D. M. Cerebral protein synthesis. II. Instability of cerebral messenger ribonucleic acid-ribosome complexes. J Biol Chem. 1968 Oct 25;243(20):5396–5409. [PubMed] [Google Scholar]


