Abstract
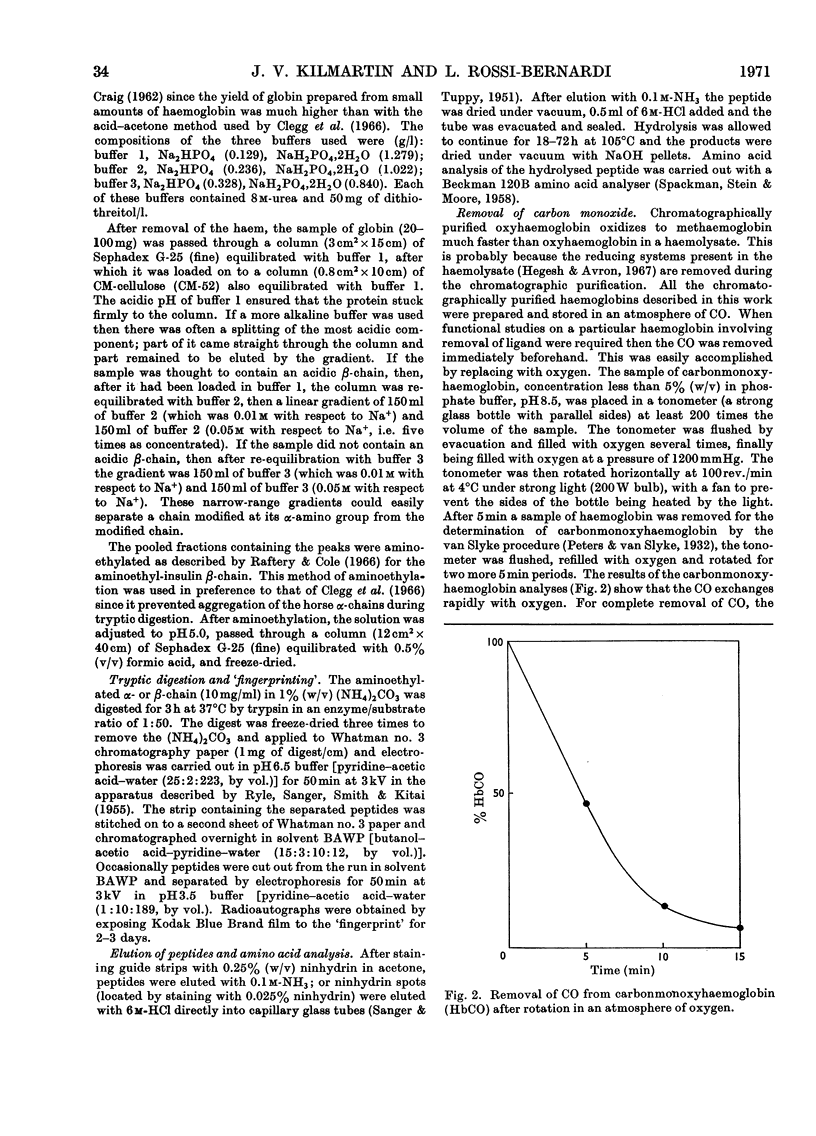
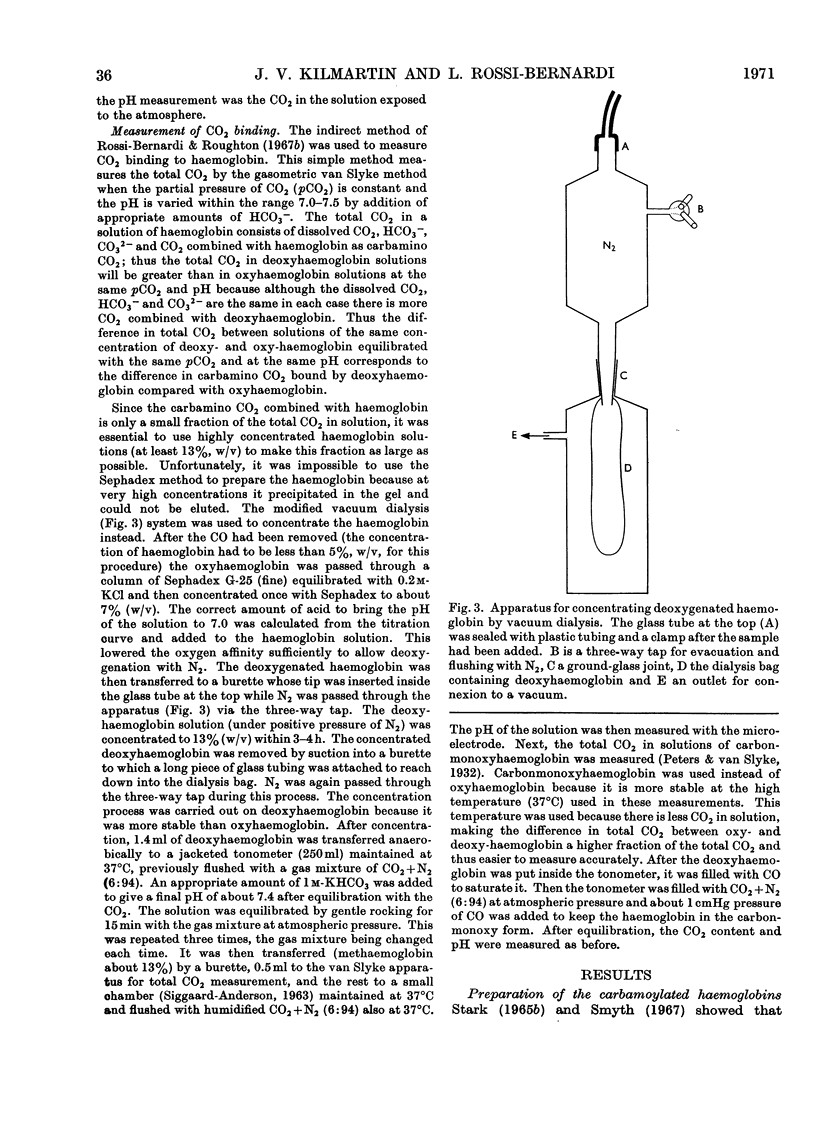
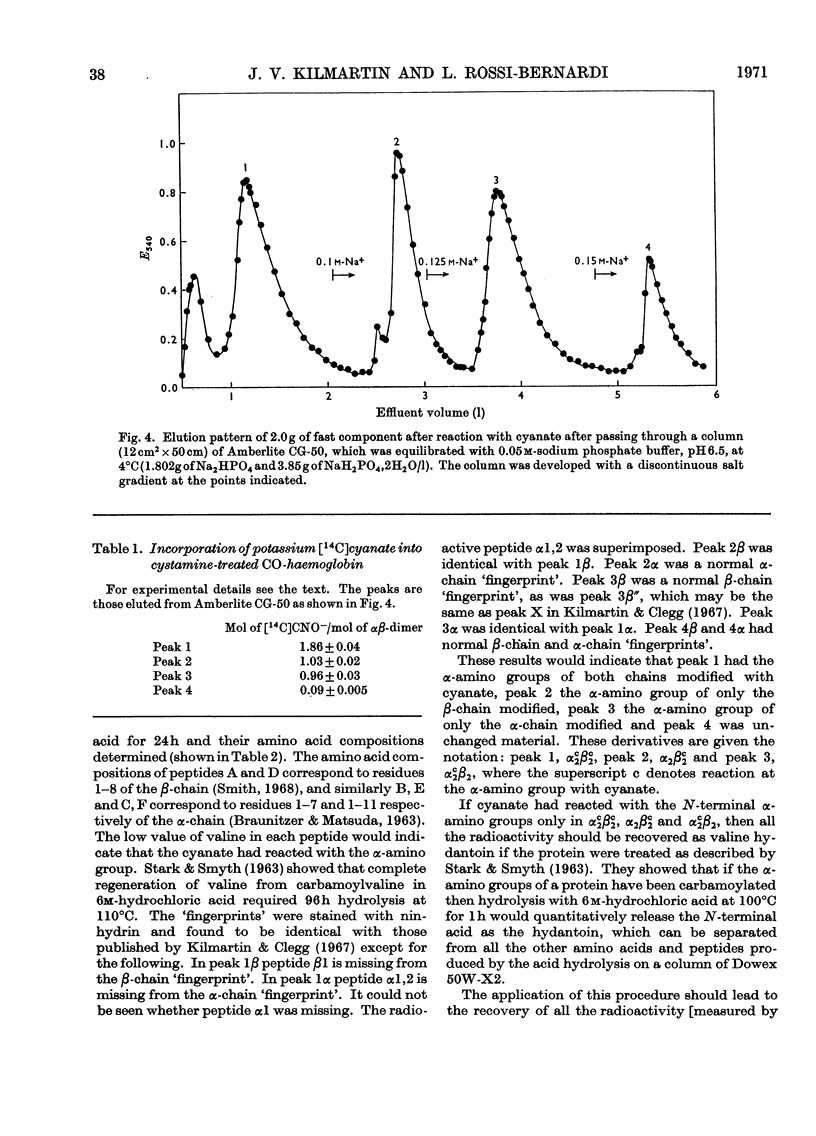
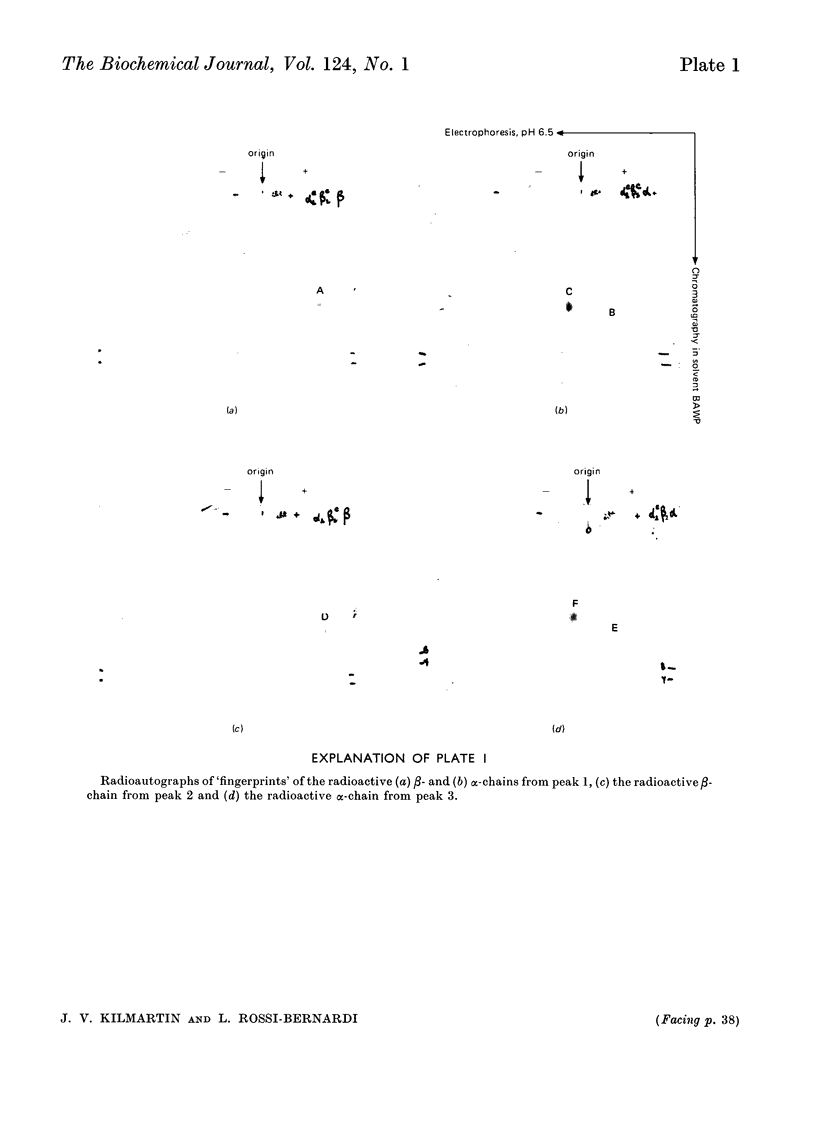
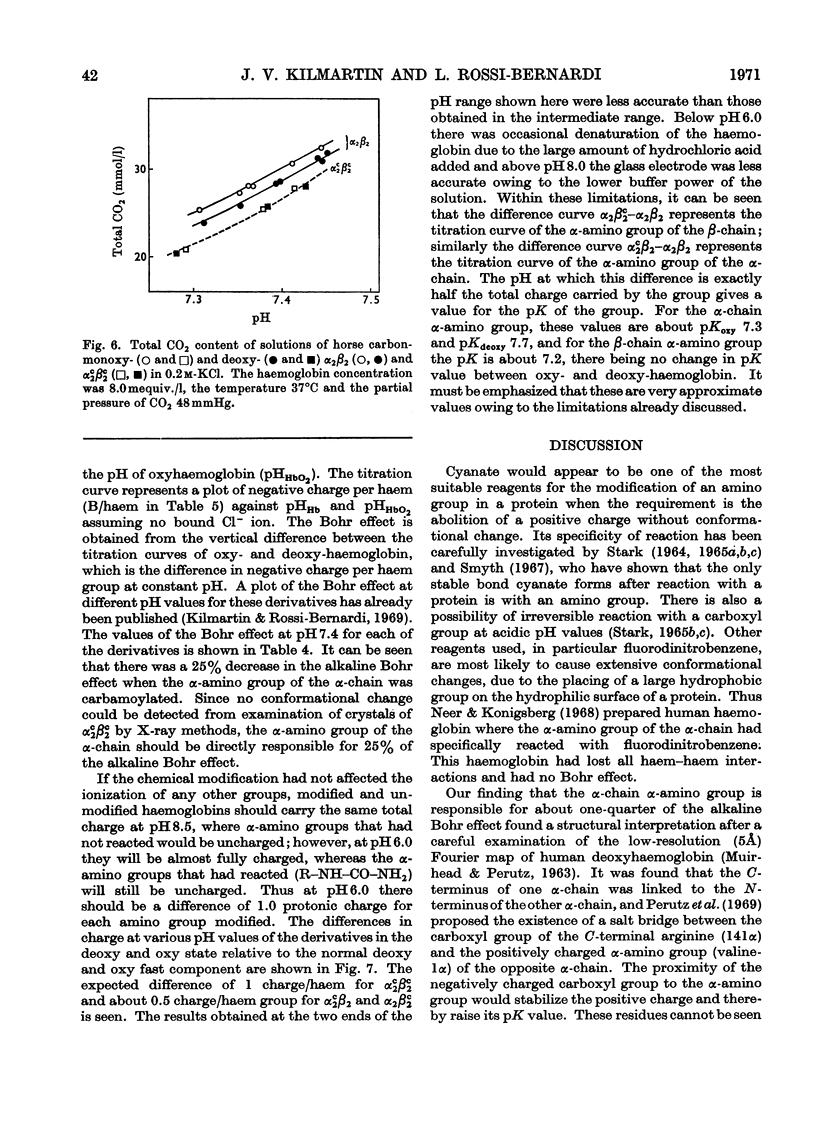
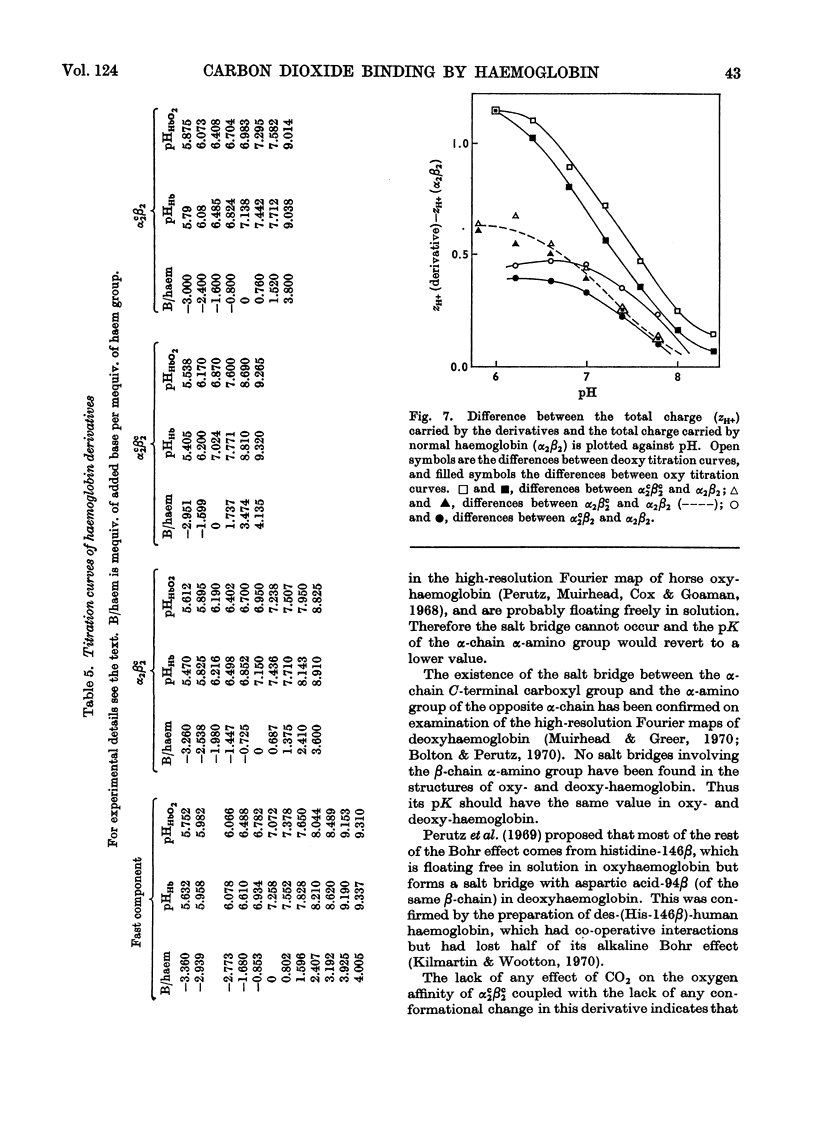
1. Three modified horse haemoglobins have been prepared: (i) αc2βc2, in which both the α-amino groups of the α- and β-chains have reacted with cyanate, (ii) αc2β2, in which the α-amino groups of the α-chains have reacted with cyanate, and (iii) α2βc2, in which the two α-amino groups of the β-chain have reacted with cyanate. 2. The values of n (the Hill constant) for αc2βc2, α2βc2 and αc2β2 were (respectively) 2.5, 2.0 and 2.6, indicating the presence of co-operative interactions between the haem groups for all derivatives. 3. In the alkaline pH range (about pH8.0) all the derivatives show the same charge as normal haemoglobin whereas in the acid pH range (about pH6.0) αc2βc2 differs by four protonic charges and αc2β2, α2βc2 by two protonic charges from normal haemoglobin, indicating that the expected number of ionizing groups have been removed. 4. αc2β2 and αc2βc2 show a 25% decrease in the alkaline Bohr effect, in contrast with α2βc2, which has the same Bohr effect as normal haemoglobin. 5. The deoxy form of αc2βc2 does not bind more CO2 than the oxy form of αc2βc2, whereas αc2β2 and α2βc2 show intermediate binding. 6. The results reported confirm the hypothesis that, under physiological conditions, haemoglobin binds CO2 through the four terminal α-amino groups and that the two terminal α-amino groups of α-chains are involved in the Bohr effect.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANGHAM A. D., LEHMANN H. Multiple haemoglobins in the horse. Nature. 1958 Jan 24;181(4604):267–268. doi: 10.1038/181267a0. [DOI] [PubMed] [Google Scholar]
- BENESCH R. E., BENESCH R., WILLIAMSON M. E. The influence of reversible oxygen binding on the interaction between hemoglobin submits. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2071–2075. doi: 10.1073/pnas.48.12.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENESCH R., BENESCH R. E. Determination of sulfhydryl and disulfide groups by specific proton displacement. Biochim Biophys Acta. 1957 Mar;23(3):643–644. doi: 10.1016/0006-3002(57)90388-8. [DOI] [PubMed] [Google Scholar]
- BRADSHAW R. A., ROGERS L. A., HILL R. L., BUETTNER-JANUSCH J. AMINO ACID COMPOSITION AND THE AMINO TERMINAL END GROUPS OF THE ALPHA- AND BETA-CHAINS OF FOUR LEMUR HEMOGLOBINS. Arch Biochem Biophys. 1965 Mar;109:571–578. doi: 10.1016/0003-9861(65)90402-9. [DOI] [PubMed] [Google Scholar]
- BRAUNITZER G., MATSUDA G. Primary structure of the alpha-chain from horse hemoglobin. J Biochem. 1963 Mar;53:262–263. doi: 10.1093/oxfordjournals.jbchem.a127691. [DOI] [PubMed] [Google Scholar]
- Bauer C. Antagonistic influence of Co2 and 2,3 diphosphoglycerate on the Bohr effect of human haemoglobin. Life Sci. 1969 Oct 15;8(20):1041–1046. doi: 10.1016/0024-3205(69)90455-x. [DOI] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E. Intracellular organic phosphates as regulators of oxygen release by haemoglobin. Nature. 1969 Feb 15;221(5181):618–622. doi: 10.1038/221618a0. [DOI] [PubMed] [Google Scholar]
- Bolton W., Perutz M. F. Three dimensional fourier synthesis of horse deoxyhaemoglobin at 2.8 Angstrom units resolution. Nature. 1970 Nov 7;228(5271):551–552. doi: 10.1038/228551a0. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Briehl R. W. The interaction of 2,3-diphosphoglycerate with various human hemoglobins. J Clin Invest. 1970 Jun;49(6):1088–1095. doi: 10.1172/JCI106324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- DAVIS N. C., SMITH E. L. Purification and some properties of prolidase of swine kidney. J Biol Chem. 1957 Jan;224(1):261–275. [PubMed] [Google Scholar]
- Forster R. E., Constantine H. P., Craw M. R., Rotman H. H., Klocke R. A. Reaction of CO2 with human hemoglobin solution. J Biol Chem. 1968 Jun 25;243(12):3317–3326. [PubMed] [Google Scholar]
- GUIDOTTI G., KONIGSBERG W. THE CHARACTERIZATION OF MODIFIED HUMAN HEMOGLOBIN. I. REACTION WITH IODOACETAMIDE AND N-ETHYLMALEIMIDE. J Biol Chem. 1964 May;239:1474–1484. [PubMed] [Google Scholar]
- HILL R. J., KONIGSBERG W., GUIDOTTI G., CRAIG L. C. The structure of human hemoglobin. I. The separation of the alpha and beta chains and their amino acid composition. J Biol Chem. 1962 May;237:1549–1554. [PubMed] [Google Scholar]
- HILL R. L., SCHMIDT W. R. The complete enzymic hydrolysis of proteins. J Biol Chem. 1962 Feb;237:389–396. [PubMed] [Google Scholar]
- HIRS C. H. W., MOORE S., STEIN W. H. A chromatographic investigation of pancreatic ribonuclease. J Biol Chem. 1953 Feb;200(2):493–506. [PubMed] [Google Scholar]
- Hegesh E., Avron M. The enzymatic reduction of ferrihemoglobin. II. Purification of a ferrihemoglobin reductase from human erythrocytes. Biochim Biophys Acta. 1967;146(2):397–408. doi: 10.1016/0005-2744(67)90224-0. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Clegg J. B. Amino-acid replacements in horse haemoglobin. Nature. 1967 Jan 21;213(5073):269–271. doi: 10.1038/213269a0. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Rossi-Bernardi L. Inhibition of CO2 combination and reduction of the Bohr effect in haemoglobin chemically modified at its alpha-amino groups. Nature. 1969 Jun 28;222(5200):1243–1246. doi: 10.1038/2221243a0. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Wootton J. F. Inhibition of Bohr effect after removal of C-terminal histidines from haemoglobin beta-chains. Nature. 1970 Nov 21;228(5273):766–767. doi: 10.1038/228766a0. [DOI] [PubMed] [Google Scholar]
- MUIRHEAD H., PERUTZ M. F. STRUCTURE OF HAEMOGLOBIN. A THREE-DIMENSIONAL FOURIER SYNTHESIS OF REDUCED HUMAN HAEMOGLOBIN AT 5-5 A RESOLUTION. Nature. 1963 Aug 17;199:633–638. doi: 10.1038/199633a0. [DOI] [PubMed] [Google Scholar]
- Muirhead H., Greer J. Three-dimensional Fourier synthesis of human deoxyhaemoglobin at 3.5 Angstrom units. Nature. 1970 Nov 7;228(5271):516–519. doi: 10.1038/228516a0. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Konigsberg W. The characterization of modified human hemoglobin. II. Reaction with 1-fluoro-2,4-dinitrobenzene. J Biol Chem. 1968 Apr 25;243(8):1966–1970. [PubMed] [Google Scholar]
- Pace M., Rossi-Bernardi L., Roughton F. J. The effect of organic phosphates on the reactions of haemoglobin and oxyhaemoglobin (carboxyhaemoglobin) with carbon dioxide. Biochem J. 1970 Oct;119(5):67P–68P. doi: 10.1042/bj1190067p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Cox J. M., Goaman L. C. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: the atomic model. Nature. 1968 Jul 13;219(5150):131–139. doi: 10.1038/219131a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Mazzarella L., Crowther R. A., Greer J., Kilmartin J. V. Identification of residues responsible for the alkaline Bohr effect in haemoglobin. Nature. 1969 Jun 28;222(5200):1240–1243. doi: 10.1038/2221240a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- RIGGS A. The metamorphosis of hemoglobin in the bullfrog. J Gen Physiol. 1951 Sep;35(1):23–40. doi: 10.1085/jgp.35.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYLE A. P., SANGER F., SMITH L. F., KITAI R. The disulphide bonds of insulin. Biochem J. 1955 Aug;60(4):541–556. doi: 10.1042/bj0600541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery M. A., Cole R. D. On the aminoethylation of proteins. J Biol Chem. 1966 Aug 10;241(15):3457–3461. [PubMed] [Google Scholar]
- Rimon S., Perlmann G. E. Carbamylation of pepsinogen and pepsin. J Biol Chem. 1968 Jul 10;243(13):3566–3572. [PubMed] [Google Scholar]
- Rossi-Bernardi L., Roughton F. J. The effect of temperature on the oxygen-linked ionizations of hemoglobin. J Biol Chem. 1967 Mar 10;242(5):784–792. [PubMed] [Google Scholar]
- Roughton F. J. Some recent work on the interactions of oxygen, carbon dioxide and haemoglobin. Biochem J. 1970 May;117(5):801–812. doi: 10.1042/bj1170801b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGER F., TUPPY H. The amino-acid sequence in the phenylalanyl chain of insulin. I. The identification of lower peptides from partial hydrolysates. Biochem J. 1951 Sep;49(4):463–481. doi: 10.1042/bj0490463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STARK G. R. ON THE REVERSIBLE REACTION OF CYANATE WITH SULFHYDRYL GROUPS AND THE DETERMINATION OF NH2-TERMINAL CYSTEINE AND CYSTINE IN PROTEINS. J Biol Chem. 1964 May;239:1411–1414. [PubMed] [Google Scholar]
- STARK G. R., SMYTH D. G. The use of cyanate for the determination of NH2-terminal residues in proteins. J Biol Chem. 1963 Jan;238:214–226. [PubMed] [Google Scholar]
- Schwartz E., Nathan D. G. New methods of counting of C-14-labeled hemoglobin and hemin. J Lab Clin Med. 1967 Nov;70(5):841–848. [PubMed] [Google Scholar]
- Smithies O. Disulfide-bond cleavage and formation in proteins. Science. 1965 Dec 17;150(3703):1595–1598. doi: 10.1126/science.150.3703.1595. [DOI] [PubMed] [Google Scholar]
- Smyth D. G. Carbamylation of amino and tyrosine hydroxyl groups. Preparation of an inhibitor of oxytocin with no intrinsic activity on the isolated uterus. J Biol Chem. 1967 Apr 10;242(7):1579–1591. [PubMed] [Google Scholar]
- Stark G. R. Reactions of cyanate with functional groups of proteins. 3. Reactions with amino and carboxyl groups. Biochemistry. 1965 Jun;4(6):1030–1036. doi: 10.1021/bi00882a008. [DOI] [PubMed] [Google Scholar]
- Stark G. R. Reactions of cyanate with functional groups of proteins. IV. Inertness of aliphatic hydroxyl groups. Formation of carbamyl- and acylhydantoins. Biochemistry. 1965 Nov;4(11):2363–2367. doi: 10.1021/bi00887a015. [DOI] [PubMed] [Google Scholar]
- Taylor J. F., Antonini E., Brunori M., Wyman J. Studies on human hemoglobin treated with various sulfhydryl reagents. J Biol Chem. 1966 Jan 10;241(1):241–248. [PubMed] [Google Scholar]
- Wallach D. F., Gail M. H., Janeway C. A. A column method for deoxygenating human hemoglobin. Anal Biochem. 1966 May;15(2):300–304. doi: 10.1016/0003-2697(66)90035-2. [DOI] [PubMed] [Google Scholar]
- van KAMPEN E., ZIJLSTRA W. G. Standardization of hemoglobinometry. II. The hemiglobincyanide method. Clin Chim Acta. 1961 Jul;6:538–544. doi: 10.1016/0009-8981(61)90145-0. [DOI] [PubMed] [Google Scholar]



