Abstract
Two xyloglucan fractions have been isolated from the cotyledons of resting white-mustard seeds, the first by extraction with hot EDTA, and the second by subsequent extraction with alkali or lithium thiocyanate. Although both appear to have the `amyloid' type of structure in which chains of (1→4)-linked β-d-glucopyranose residues carry d-xylose-rich side chains through position 6, these side chains are rather different in structure in the two polysaccharide fractions, and the second or `insoluble' xyloglucan has fewer of them. The side chains in both polysaccharides are also different from those in other seed amyloids, especially in having xylose linked through positions 3 and 4 (instead of through position 2 as usual) and in containing fucose residues. Both polysaccharides show the characteristic blue `amyloid' colour with iodine in the presence of sodium sulphate, and it is suggested that this arises by the interaction of iodine molecules and possibly iodide ions within the interstices between aggregated xyloglucan chains. `Soluble' xyloglucan is metabolized during germination and is presumed to have a reserve function. `Insoluble' xyloglucan is metabolized less completely over the period studied but its lack of turnover during cell-wall differentiation indicates that it also is a reserve. These and other β-(1→4)-linked reserve polysaccharides of seeds might also have a structural function which is of particular value for the survival of the dormant seed.
Full text
PDF

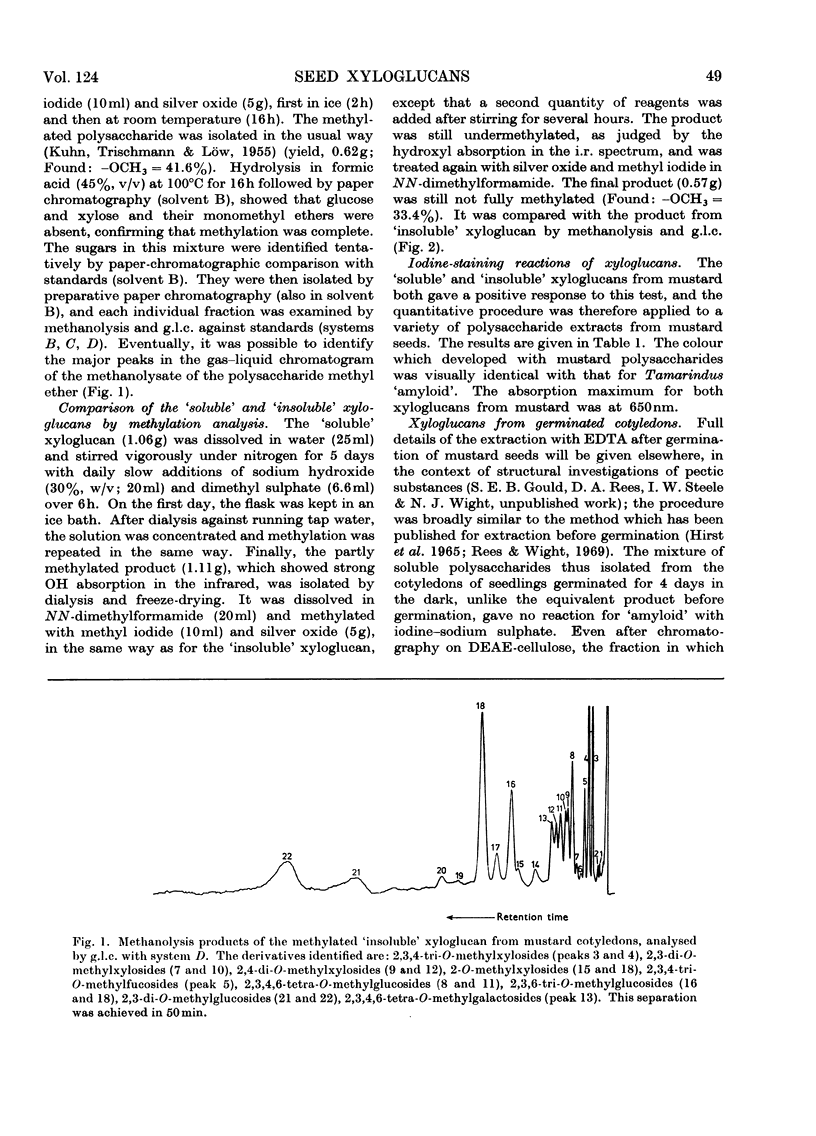
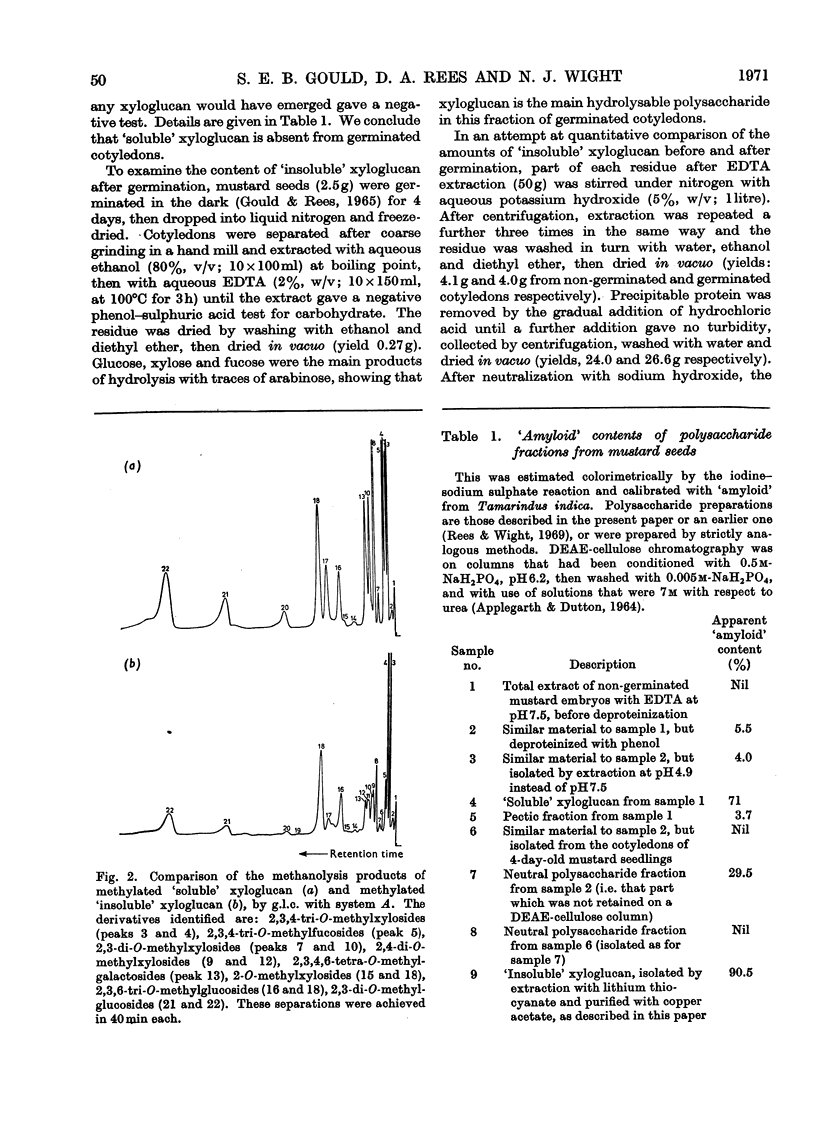



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPLEGARTH D. A., DUTTON G. G. COLUMN CHROMATOGRAPHY OF POLYSACCHARIDES IN THE PRESENCE OF UREA. J Chromatogr. 1964 Jul;15:246–246. doi: 10.1016/s0021-9673(01)82775-8. [DOI] [PubMed] [Google Scholar]
- Aspinall G. O. Gums and mucilages. Adv Carbohydr Chem Biochem. 1969;24:333–379. doi: 10.1016/s0065-2318(08)60353-4. [DOI] [PubMed] [Google Scholar]
- Aspinall G. O., Molloy J. A., Craig J. W. Extracellular polysaccharides from suspension-cultured sycamore cells. Can J Biochem. 1969 Nov;47(11):1063–1070. doi: 10.1139/o69-170. [DOI] [PubMed] [Google Scholar]
- Gaillard B. D., Bailey R. W. Reaction with iodine of polysaccharides dissolved in strong calcium chloride solution. Nature. 1966 Oct 8;212(5058):202–203. doi: 10.1038/212202a0. [DOI] [PubMed] [Google Scholar]
- HIRST E. L., REES D. A., RICHARDSON N. G. SEED POLYSACCHARIDES AND THEIR ROLE IN GERMINATION. A SURVEY OF THE POLYSACCHARIDE COMPONENTS OF MUSTARD SEEDS WITH SPECIAL REFERENCE TO THE EMBRYOS. Biochem J. 1965 May;95:453–458. doi: 10.1042/bj0950453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. A., Richardson N. G. Polysaccharides in germination. Occurrence, fine structure, and possible biological role of the pectic araban in white mustard cotyledons. Biochemistry. 1966 Oct;5(10):3099–3107. doi: 10.1021/bi00874a003. [DOI] [PubMed] [Google Scholar]
- Rees D. A., Steele I. W. Polysaccharides in germination. Physical characterization of the pectic araban of white mustard cotyledons. Biochemistry. 1966 Oct;5(10):3108–3110. doi: 10.1021/bi00874a004. [DOI] [PubMed] [Google Scholar]
- Rees D. A., Wight N. J. Molecular cohesion in plant cell walls. Methylation analysis of pectic polysaccharides from the cotyledons of white mustard. Biochem J. 1969 Nov;115(3):431–439. doi: 10.1042/bj1150431. [DOI] [PMC free article] [PubMed] [Google Scholar]


