Abstract
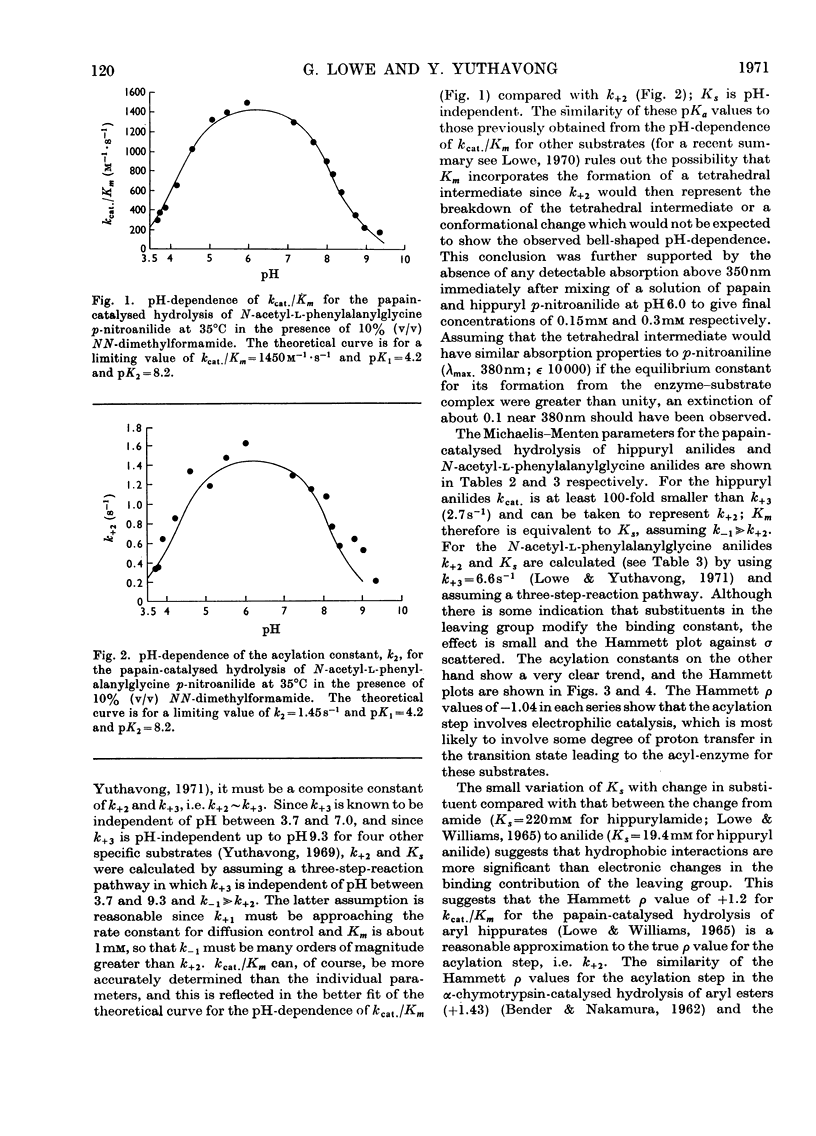
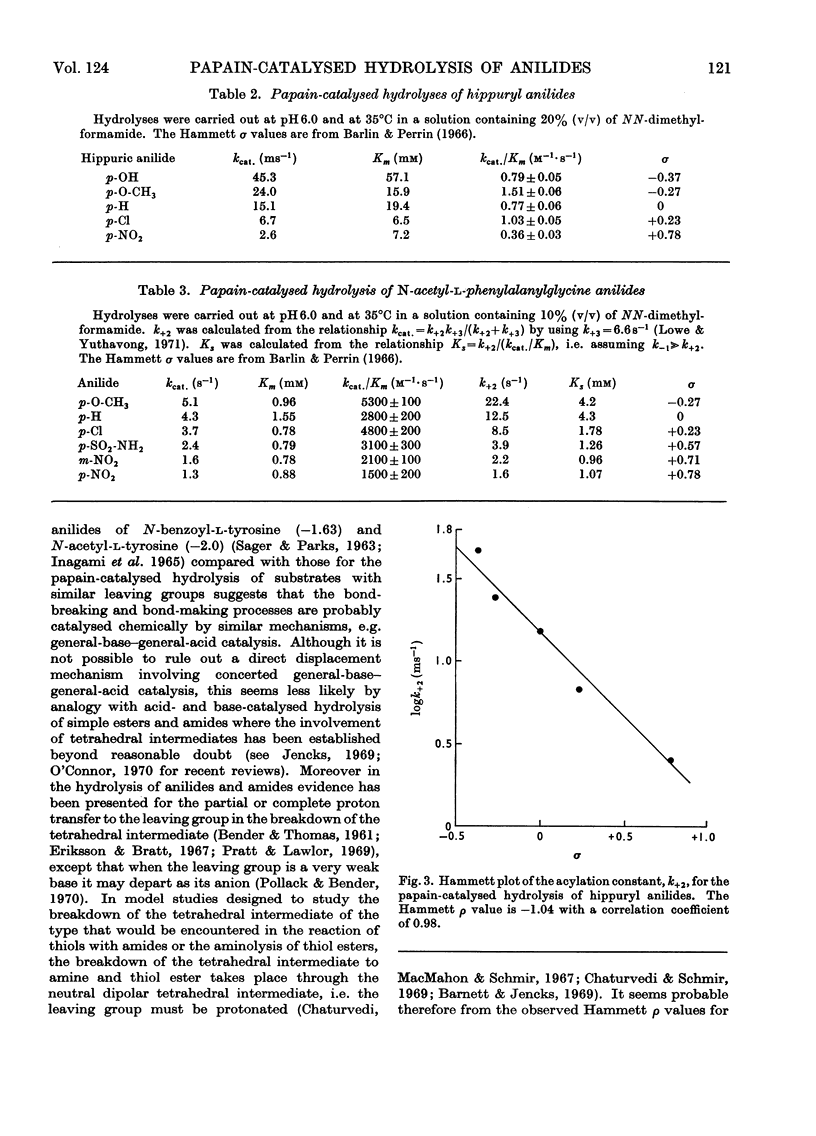
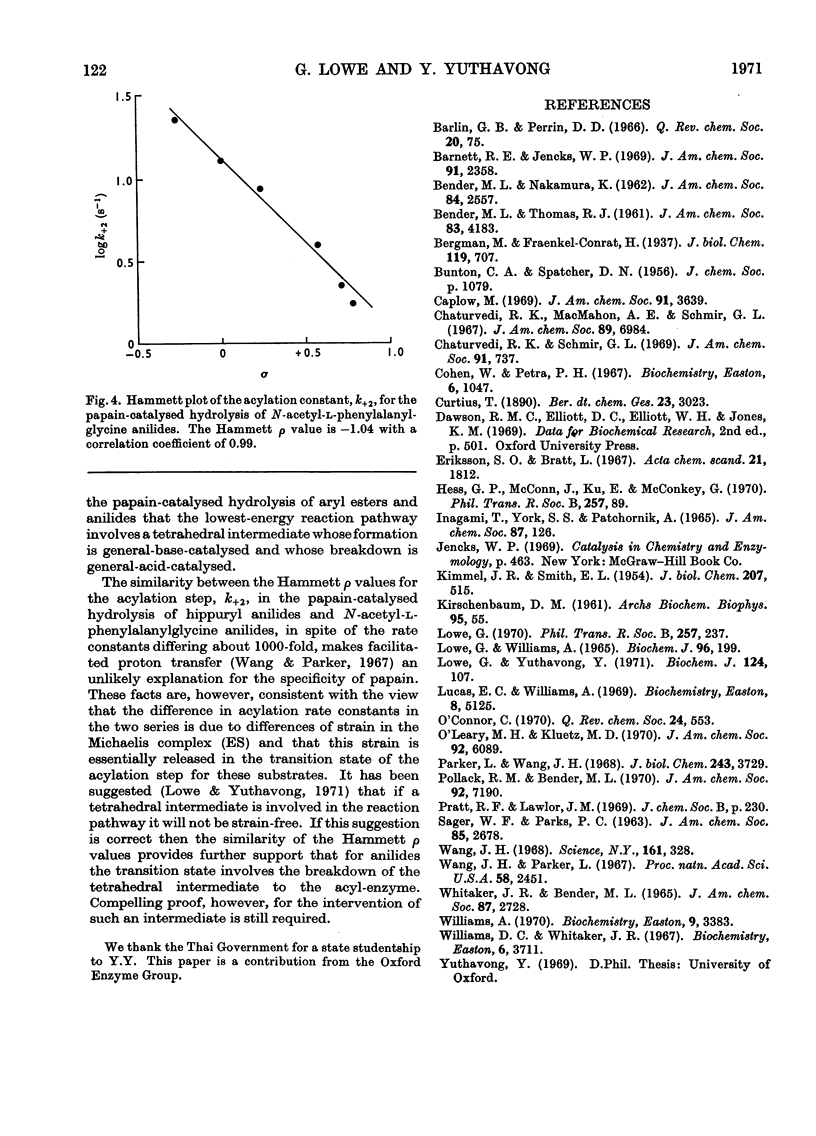
The pH-dependence of the Michaelis–Menten parameters for the papain-catalysed hydrolysis of N-acetyl-l-phenylalanylglycine p-nitroanilide was determined. The equilibrium binding constant, Ks, is independent of pH between 3.7 and 9.3, whereas the acylation constant, k+2, shows bell-shaped pH-dependence with apparent pKa values of 4.2 and 8.2. The effect of substituents in the leaving group on the acylation constant of the papain-catalysed hydrolysis of hippuryl anilides and N-acetyl-l-phenylalanylglycine anilides gives rise in both series to a Hammett ρ value of −1.04. This indicates that the enzyme provides electrophilic, probably general-acid, catalysis, as well as the nucleophilic or general-base catalysis previously found. A mechanism involving a tetrahedral intermediate whose formation is general-base-catalysed and whose breakdown is general-acid-catalysed seems most likely. The similarity of the Hammett ρ values appears to exclude facilitated proton transfer as a means through which the specificity of papain is expressed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caplow M. Chymotrypsin catalysis. Evidence for a new intermediate. J Am Chem Soc. 1969 Jun 18;91(13):3639–3645. doi: 10.1021/ja01041a037. [DOI] [PubMed] [Google Scholar]
- Cohen W., Petra P. H. A kinetic analysis of the papain-catalyzed hydrolysis of alpha-N-benzoyl-L-citrulline methyl ester. Biochemistry. 1967 Apr;6(4):1047–1053. doi: 10.1021/bi00856a013. [DOI] [PubMed] [Google Scholar]
- Inagami T., York S. S., Patchornik A. An electrophilic mechanism in the chymotrypsin-catalyzed hydrolysis of anilide substrates. J Am Chem Soc. 1965 Jan 5;87(1):126–127. doi: 10.1021/ja01079a027. [DOI] [PubMed] [Google Scholar]
- KIMMEL J. R., SMITH E. L. Crystalline papain. I. Preparation, specificity, and activation. J Biol Chem. 1954 Apr;207(2):515–531. [PubMed] [Google Scholar]
- LOWE G., WILLIAMS A. PAPAIN-CATALYSED HYDROLYSIS OF SOME HIPPURIC ESTERS. A NEW MECHANISM FOR PAPAIN-CATALYSED HYDROLYSIS. Biochem J. 1965 Jul;96:199–204. doi: 10.1042/bj0960199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe G., Yuthavong Y. Kinetic specificity in papain-catalysed hydrolyses. Biochem J. 1971 Aug;124(1):107–115. doi: 10.1042/bj1240107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas E. C., Williams A. The pH dependencies of individual rate constants in papain-catalyzed reactions. Biochemistry. 1969 Dec;8(12):5125–5135. doi: 10.1021/bi00840a067. [DOI] [PubMed] [Google Scholar]
- O'Leary M. H., Kluetz M. D. Identification of the rate-limiting step in the chymotrypsin-catalyzed hydrolysis of N-acetyl-L-tryptophanamide. J Am Chem Soc. 1970 Oct 7;92(20):6089–6090. doi: 10.1021/ja00723a062. [DOI] [PubMed] [Google Scholar]
- Parker L., Wang J. H. On the mechanism of action at the acylation step of the alpha-chymotrypsin-catalyzed hydrolysis of anilides. J Biol Chem. 1968 Jul 10;243(13):3729–3734. [PubMed] [Google Scholar]
- WHITAKER J. R., BENDER M. L. KINETICS OF PAPAIN-CATALYZED HYDROLYSIS OF ALPHA-N-BENZOYL-L-ARGININE ETHYL ESTER AND ALPHA-N-BENZOYL-L-ARGININAMIDE. J Am Chem Soc. 1965 Jun 20;87:2728–2737. doi: 10.1021/ja01090a034. [DOI] [PubMed] [Google Scholar]
- Wang J. H., Parker L. Pretransition-state protonation and the rate of chymotrypsin catalysis. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2451–2454. doi: 10.1073/pnas.58.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. Chymotrypsin-catalyzed phenyl ester hydrolysis. Evidence for electrophilic assistance on carbonyl oxygen. Biochemistry. 1970 Aug 18;9(17):3383–3390. doi: 10.1021/bi00819a014. [DOI] [PubMed] [Google Scholar]
- Williams D. C., Whitaker J. R. Kinetics of papain-catalyzed hydrolyses of neutral substrates. Biochemistry. 1967 Dec;6(12):3711–3717. doi: 10.1021/bi00864a013. [DOI] [PubMed] [Google Scholar]


