Abstract
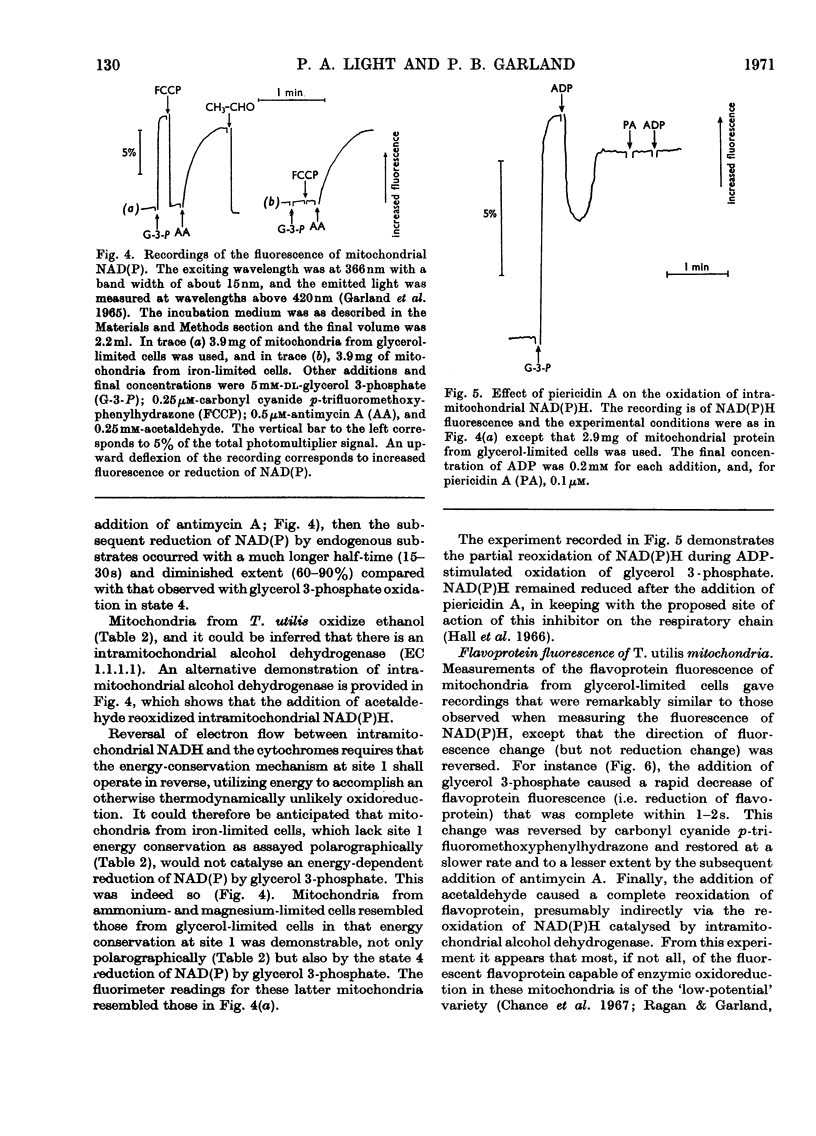
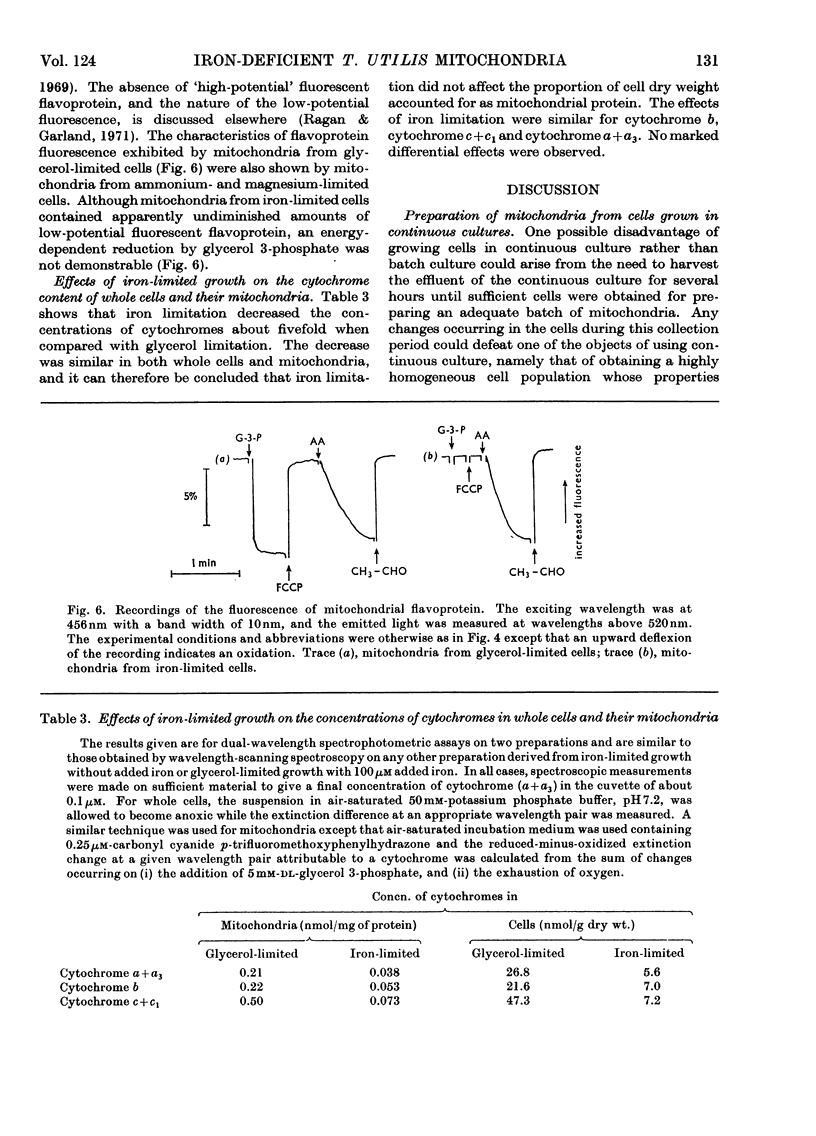
1. Mitochondria prepared from Torulopsis utilis grown in a chemostat with iron-limited growth were found to lack energy conservation but not electron flow in that segment of the respiratory chain leading from intramitochondrial NADH to the cytochromes [i.e. the site 1 segment (Lehninger, 1964)]. 2. Site 1 energy conservation was present in mitochondria prepared from cells grown under conditions of limitation by glycerol, ammonium and magnesium. Phosphate-limited growth resulted in mitochondrial preparations without respiratory control. 3. Mitochondria from cells grown under conditions of iron limitation were insensitive to the respiratory inhibitor piericidin A, whereas sensitivity was present in mitochondria prepared from glycerol-, ammonium-, magnesium- or phosphate-limited cells. 4. These observations are considered to provide indirect evidence for a role of non-haem iron in the mechanism of energy conservation and also piericidin A sensitivity in T. utilis mitochondria. 5. A readily constructed and inexpensive pH-measuring and -controlling circuit is described for use with continuous-culture apparatus.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackrell B. A., Jones C. W. The respiratory system of Azotobacter vinelandii. 2. Oxygen effects. Eur J Biochem. 1971 May 11;20(1):29–35. doi: 10.1111/j.1432-1033.1971.tb01358.x. [DOI] [PubMed] [Google Scholar]
- Bois R., Estabrook R. W. Nonheme iron protein as a possible site of rotenone inhibition of mitochondrial NADH dehydrogenase. Arch Biochem Biophys. 1969 Jan;129(1):362–369. doi: 10.1016/0003-9861(69)90187-8. [DOI] [PubMed] [Google Scholar]
- Butow R. A., Racker E. On the mechanism of respiratory control. J Gen Physiol. 1965 Sep;49(1 Suppl):149–162. doi: 10.1085/jgp.49.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butow R. A., Zeydel M. The isolation of an antimycin-resistant mutant of orulopsis utilis. J Biol Chem. 1968 May 25;243(10):2545–2549. [PubMed] [Google Scholar]
- CHANCE B., BALTSCHEFFSKY H. Respiratory enzymes in oxidative phosphorylation. VII. Binding of intramitochondrial reduced pyridine nucleotide. J Biol Chem. 1958 Sep;233(3):736–739. [PubMed] [Google Scholar]
- CHANCE B., HOLLUNGER G. Energy-linked reduction of mitochondrial pyridine nucleotide. Nature. 1960 Mar 5;185:666–672. doi: 10.1038/185666a0. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- CRANE F. L., GLENN J. L., GREEN D. E. Studies on the electron transfer system. IV. The electron transfer particle. Biochim Biophys Acta. 1956 Dec;22(3):475–487. doi: 10.1016/0006-3002(56)90058-0. [DOI] [PubMed] [Google Scholar]
- Chance B., Ernster L., Garland P. B., Lee C. P., Light P. A., Ohnishi T., Ragan C. I., Wong D. Flavoproteins of the mitochondrial respiratory chain. Proc Natl Acad Sci U S A. 1967 May;57(5):1498–1505. doi: 10.1073/pnas.57.5.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell J. B. The effects of 2,4-dinitrophenol on mitochondrial oxidations. Biochem J. 1964 Feb;90(2):237–248. doi: 10.1042/bj0900237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell J. B. The oxidation of citrate, isocitrate and cis-aconitate by isolated mitochondria. Biochem J. 1964 Feb;90(2):225–237. doi: 10.1042/bj0900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg R. A., Garland P. B. Non-haem iron and the dissociation of piericidin A sensitivity from site 1 energy conservation in mitochondria from Torulopsis utilis. Biochem J. 1971 Aug;124(1):135–151. doi: 10.1042/bj1240135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg R. A., Ragan C. I., Haddock B. A., Light P. A., Garland P. B., Swann J. C., Bray R. C. Inter-relationships between mitochondrial energy conservation at site I, piericidin a sensitivity, and EPR spectra in torulopsis utilis. FEBS Lett. 1969 Nov 12;5(3):207–210. doi: 10.1016/0014-5793(69)80333-9. [DOI] [PubMed] [Google Scholar]
- DUELL E. A., INOUE S., UTTER M. F. ISOLATION AND PROPERTIES OF INTACT MITOCHONDRIA FROM SPHEROPLASTS OF YEAST. J Bacteriol. 1964 Dec;88:1762–1773. doi: 10.1128/jb.88.6.1762-1773.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaille J., Vignais P. M., Vignais P. V. Apport d'un nouvel inhibiteur, la fuscine, dans l'étude des chaînes respiratoires de mitochondries animals et de mitochondries de levures. Eur J Biochem. 1970 Apr;13(3):416–427. doi: 10.1111/j.1432-1033.1970.tb00945.x. [DOI] [PubMed] [Google Scholar]
- Fukami M. H., Light P. A., Garland P. B. Changes of mitochondrial NADH(2) oxidation pathways in Torulopsis utilis grown on acetate. FEBS Lett. 1970 Apr 2;7(2):132–134. doi: 10.1016/0014-5793(70)80138-7. [DOI] [PubMed] [Google Scholar]
- GARLAND P. B., RANDLE P. J. A rapid enzymatic assay for glycerol. Nature. 1962 Dec 8;196:987–988. doi: 10.1038/196987a0. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Shepherd D., Yates D. W. Steady-state concentrations of coenzyme A, acetyl-coenzyme A and long-chain fatty acyl-coenzyme A in rat-liver mitochondria oxidizing palmitate. Biochem J. 1965 Nov;97(2):587–594. doi: 10.1042/bj0970587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERBERT D., ELSWORTH R., TELLING R. C. The continuous culture of bacteria; a theoretical and experimental study. J Gen Microbiol. 1956 Jul;14(3):601–622. doi: 10.1099/00221287-14-3-601. [DOI] [PubMed] [Google Scholar]
- Haddock B. A., Garland P. B. Effect of sulphate-limited growth on mitochondrial electron transfer and energy conservation between reduced nicotinamide-adenine dinucleotide and the cytochromes in Torulopsis utilis. Biochem J. 1971 Aug;124(1):155–170. doi: 10.1042/bj1240155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C., Wu M., Crane F. L., Takahashi H., Tamura S., Folkers K. Piericidin A: a new inhibitor of mitochondrial electron transport. Biochem Biophys Res Commun. 1966 Nov 22;25(4):373–377. doi: 10.1016/0006-291x(66)90214-2. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Stempel K. E. Resolution of complex I (DPNH-coenzyme Q reductase) of the mitochondrial electron transfer system. Biochem Biophys Res Commun. 1967 Feb 8;26(3):301–308. doi: 10.1016/0006-291x(67)90122-2. [DOI] [PubMed] [Google Scholar]
- Herbert D., Phipps P. J., Tempest D. W. The chemostat: design and instrumentation. Lab Pract. 1965 Oct;14(10):1150–1161. [PubMed] [Google Scholar]
- Imai K., Asano A., Sato R. Oxidative phosphorylation in Micrococcus denitrificans. IV. Further characterization of electron-transfer pathway and phosphorylation activity in NADH oxidation. J Biochem. 1968 Feb;63(2):207–218. doi: 10.1093/oxfordjournals.jbchem.a128763. [DOI] [PubMed] [Google Scholar]
- Imai K., Asano A., Sato R. Oxidative phosphorylation in Micrococcus denitrificans. V. Effects of iron deficiency on respiratory components and oxidative phosphorylation. J Biochem. 1968 Feb;63(2):219–225. doi: 10.1093/oxfordjournals.jbchem.a128764. [DOI] [PubMed] [Google Scholar]
- KOTELNIKOVA A. V., ZVIAGILSKAIA R. A. OKISLITEL'NOE FOSFORILIROVANIE V SUBKLETOCHNYKH PREPARATAKH IZ DROZHZHE I ENDOMYCES MAGNUSII. Biokhimiia. 1963 Sep-Oct;28:879–887. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Light P. A., Ragan C. I., Clegg R. A., Garland P. B. Iron-limited growth of torulopsis utilis, and the reversible loss of mitochondrial energy conservation at site 1 and of sensitivity to rotenone and piericidin A. FEBS Lett. 1968 Jul;1(1):4–8. doi: 10.1016/0014-5793(68)80004-3. [DOI] [PubMed] [Google Scholar]
- Moss F. J., Rickard P. A., Beech G. A., Bush F. E. The response by microorganisms to steady state growth in controlled concentrations of oxygen and glucose. I. Candida utilis. Biotechnol Bioeng. 1969 Jul;11(4):561–580. doi: 10.1002/bit.260110404. [DOI] [PubMed] [Google Scholar]
- Ohnishi T., Kawaguchi K., Hagihara B. Preparation and some properties of yeast mitochondria. J Biol Chem. 1966 Apr 25;241(8):1797–1806. [PubMed] [Google Scholar]
- Ohnishi T., Schleyer H., Chance B. Studies on non-heme iron proteins and the piericidin A binding site of submitochondrial particles from Candida utilis cells grown in media of varying iron concentrations. Biochem Biophys Res Commun. 1969 Aug 7;36(3):487–493. doi: 10.1016/0006-291x(69)90591-9. [DOI] [PubMed] [Google Scholar]
- Ohnishi T., Sottocasa G., Ernster L. Current approaches to the mechanism of energy-coupling in the respiratory chain. Studies with yeast mitochondria. Bull Soc Chim Biol (Paris) 1966;48(11):1189–1203. [PubMed] [Google Scholar]
- Onishi T. Induction of the site I phosphorylation in vivo in Saccharomyces carlsbergensis. Biochem Biophys Res Commun. 1970 Oct 23;41(2):344–352. doi: 10.1016/0006-291x(70)90510-3. [DOI] [PubMed] [Google Scholar]
- Onishi T., Kröger A., Heldt H. W., Pfaff E., Klingenberg M. The response of the respiratory chain and adenine nucleotide system to oxidative phosphorylation in yeast mitochondria. Eur J Biochem. 1967 May;1(3):301–311. doi: 10.1007/978-3-662-25813-2_41. [DOI] [PubMed] [Google Scholar]
- Palmer G., Horgan D. J., Tisdale H., Singer T. P., Beinert H. Studies on the respiratory chain-linked reduced nicotinamide adenine dinucleotide dehydrogenase. XIV. Location of the sites of inhibition of rotenone, barbiturates, and piericidin by means of electron paramagnetic resonance spectroscopy. J Biol Chem. 1968 Feb 25;243(4):844–847. [PubMed] [Google Scholar]
- RAJAGOPALAN K. V., HANDLER P. THE ABSORPTION SPECTRA OF IRON-FLAVOPROTEINS. J Biol Chem. 1964 May;239:1509–1514. [PubMed] [Google Scholar]
- RIESKE J. S., ZAUGG W. S., HANSEN R. E. STUDIES ON THE ELECTRON TRANSFER SYSTEM. LIX. DISTRIBUTION OF IRON AND OF THE COMPONENT GIVING AN ELECTRON PARAMAGNETIC RESONANCE SIGNAL AT G = 1.90 IN SUBFRACTIONS OF COMPLEX 3. J Biol Chem. 1964 Sep;239:3023–3030. [PubMed] [Google Scholar]
- Ragan C. I., Garland P. B. Spectroscopic studies of flavoproteins and non-haem iron proteins of submitochondrial particles of Torulopsis utilis modified by iron- and sulphate-limited growth in continuous culture. Biochem J. 1971 Aug;124(1):171–187. doi: 10.1042/bj1240171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragan C. I., Garland P. B. The intra-mitochondrial localization of flavoproteins previously assigned to the respiratory chain. Eur J Biochem. 1969 Oct;10(3):399–410. doi: 10.1111/j.1432-1033.1969.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Schatz G., Racker E., Tyler D. D., Gonze J., Estabrook R. W. Studies of the DPNH-cytochrome b segment of the respiratory chain of baker's yeast. Biochem Biophys Res Commun. 1966 Mar 8;22(5):585–590. doi: 10.1016/0006-291x(66)90315-9. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI N., SUZUKI A., TAMURA S. STRUCTURE OF PIERCIDIN A. J Am Chem Soc. 1965 May 5;87:2066–2068. doi: 10.1021/ja01087a050. [DOI] [PubMed] [Google Scholar]
- Tempest D. W., Dicks J. W., Meers J. L. Magnesium-limited growth of Bacillus subtilis, in pure and mixed cultures, in a chemostat. J Gen Microbiol. 1967 Oct;49(1):139–147. doi: 10.1099/00221287-49-1-139. [DOI] [PubMed] [Google Scholar]
- Tempest D. W., Herbert D. Effect of dilution rate and growth-limiting substrate on the metabolic activity of Torula utilis cultures. J Gen Microbiol. 1965 Oct;41(1):143–150. doi: 10.1099/00221287-41-1-143. [DOI] [PubMed] [Google Scholar]
- Vanneste W. H. Molecular proportion of the fixed cytochrome components of the respiratory chain of Keilin-Hartree particles and beef heart mitochondria. Biochim Biophys Acta. 1966 Jan 11;113(1):175–178. doi: 10.1016/s0926-6593(66)80132-7. [DOI] [PubMed] [Google Scholar]
- Ware G. C., Thompson A. S., Light P. A. A non-metallic chemostat for micro-organisms requiring controlled trace metal concentrations. Lab Pract. 1970 Feb;19(2):181–182. [PubMed] [Google Scholar]
- Yamashita S., Racker E. Reconstitution of the mitochondrial oxidation chain from individual components. J Biol Chem. 1968 May 10;243(9):2446–2447. [PubMed] [Google Scholar]
- von Jagow G., Klingenberg M. Pathways of hydrogen in mitochondria of Saccharomyces carlsbergensis. Eur J Biochem. 1970 Feb;12(3):583–592. doi: 10.1111/j.1432-1033.1970.tb00890.x. [DOI] [PubMed] [Google Scholar]


