Abstract
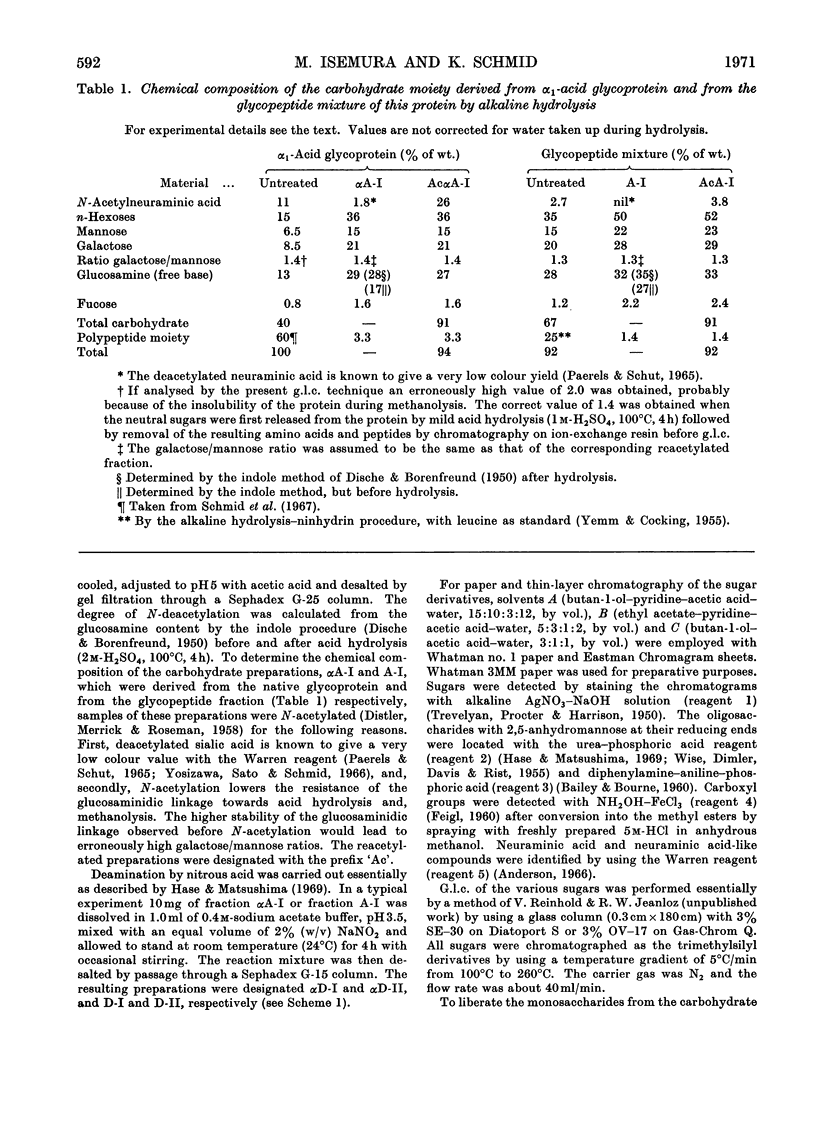
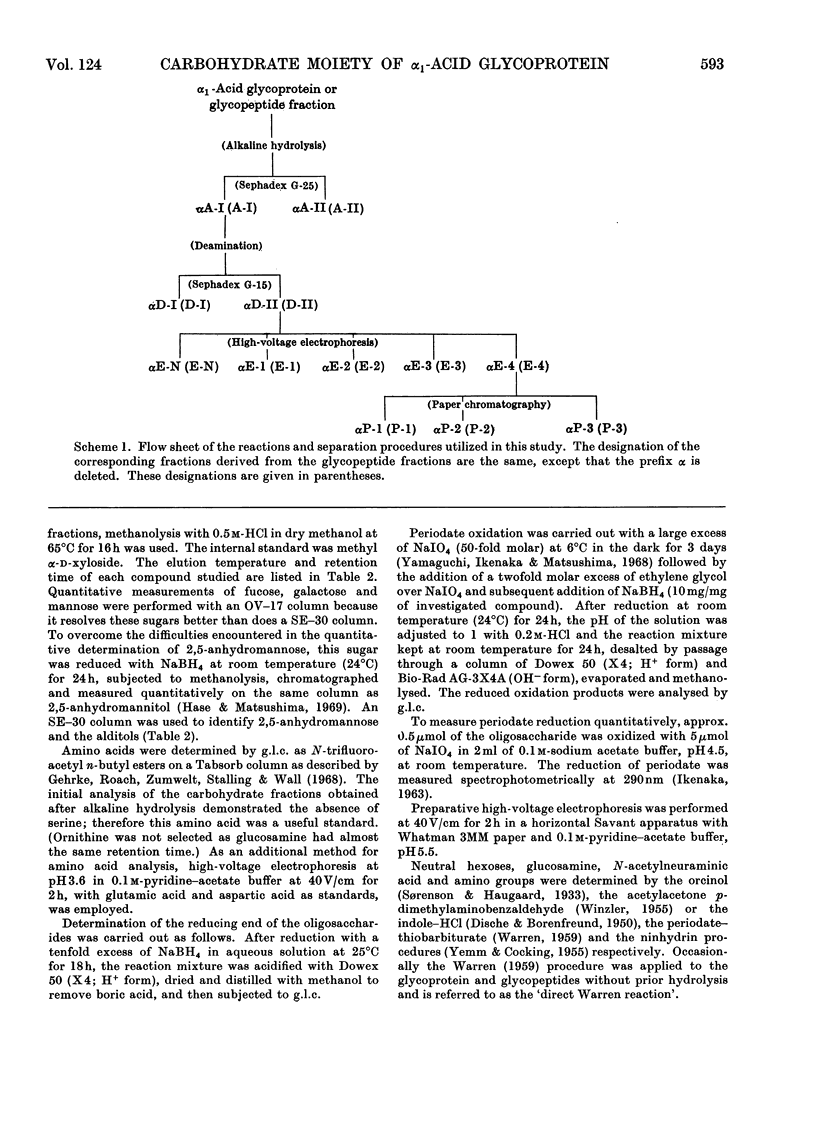
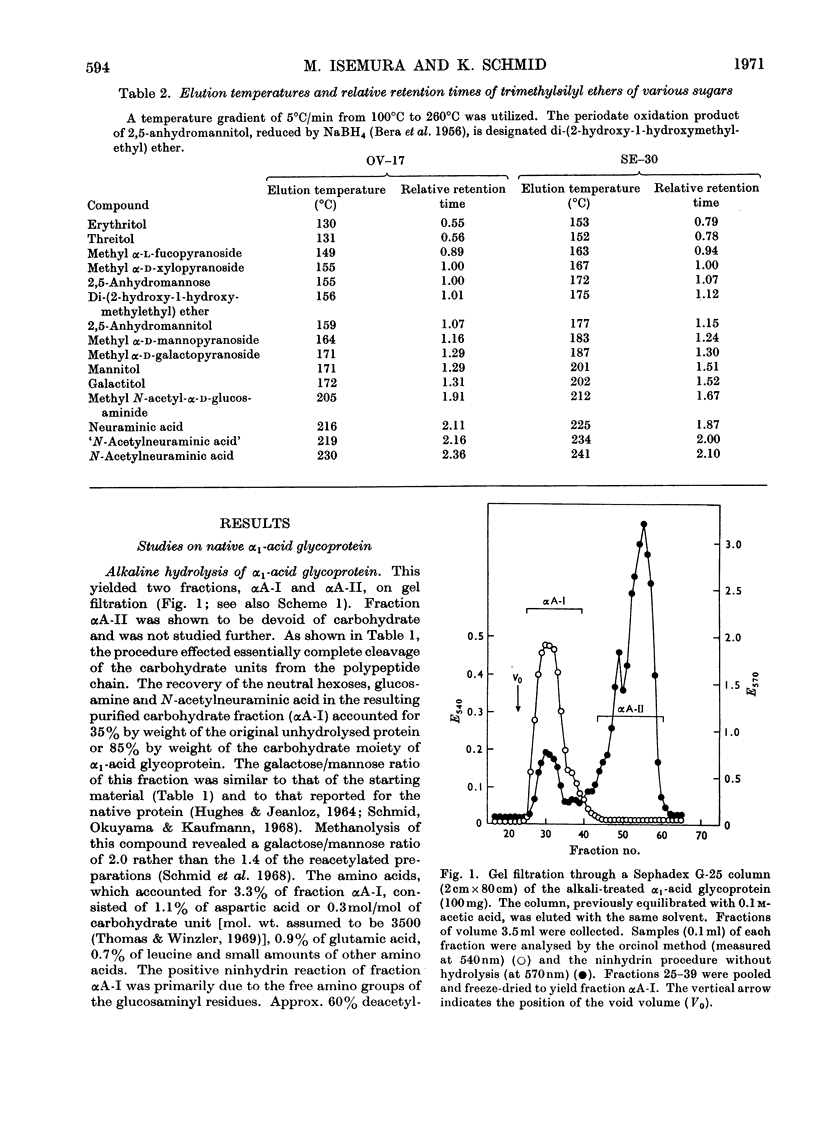
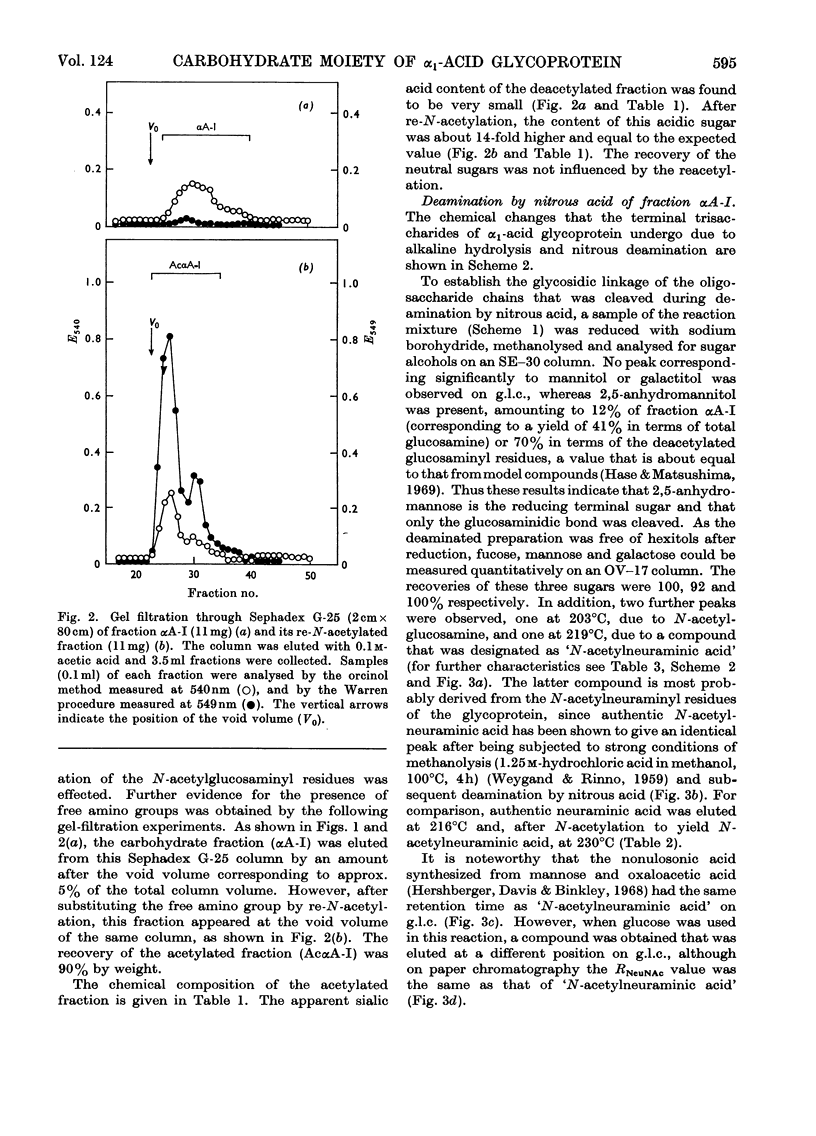
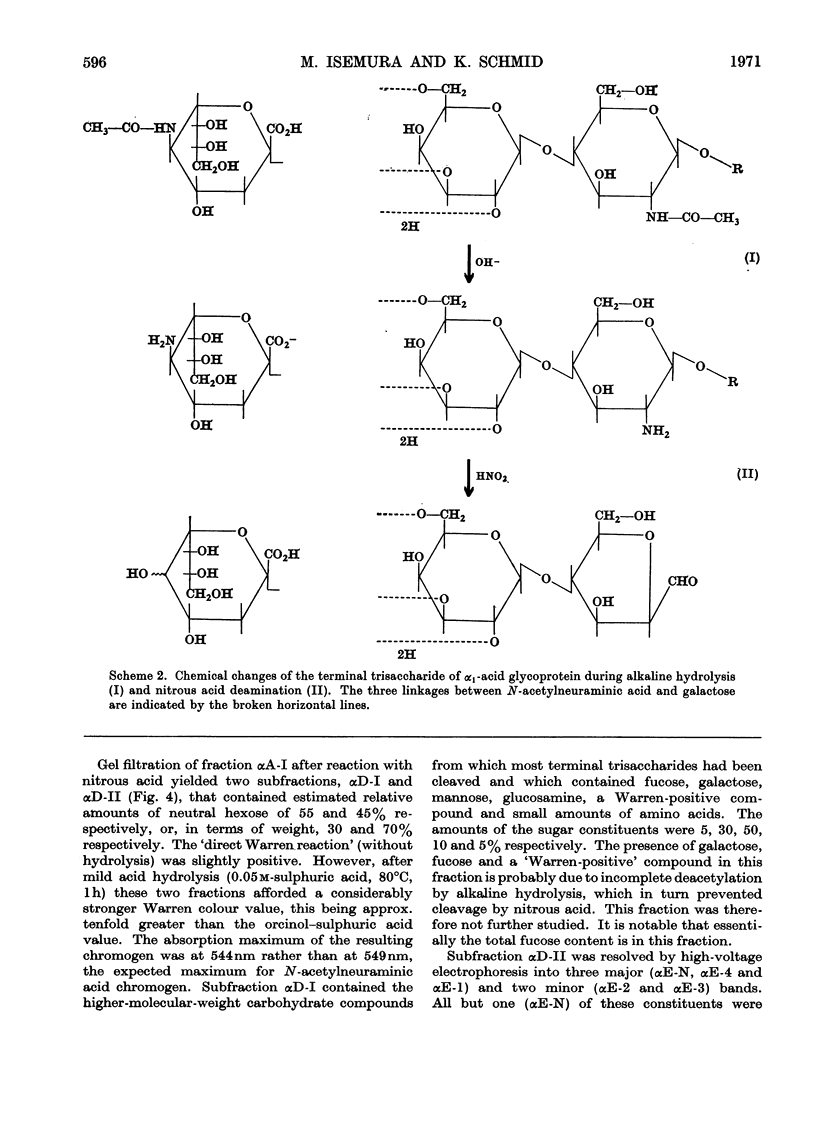
Alkaline hydrolysis followed by deamination with nitrous acid was applied for the first time to a glycoprotein, human plasma α1-acid glycoprotein (orosomucoid). This procedure, which specifically cleaves the glycosaminidic bonds, yielded well-defined oligosaccharides. The trisaccharides, which were obtained from the native protein, consisted of a sialic acid derivative, galactose and 2,5-anhydromannose. The linkage between galactose and 2,5-anhydromannose is most probably a (1→4)-glycosidic bond. A hitherto unknown linkage between N-acetylneuraminic acid and galactose was also established, namely a (2→2)-linkage. The three linkages between sialic acid and galactose described in this paper appear to be about equally resistant to mild acid hydrolysis. The disaccharide that was derived from the desialized glycoprotein consisted of galactose and 2,5-anhydromannose. Evidence was obtained for the presence of a new terminal sialyl→N-acetylglucosamine disaccharide accounting for approximately 1mol/mol of protein. The presence of this disaccharide may explain the relatively severe requirements for the complete acid hydrolysis of the sialyl residues. The present study indicates that alkaline hydrolysis followed by nitrous acid deamination in conjunction with gas–liquid chromatography will afford relatively rapid determination of the partial structure of the complex carbohydrate moiety of glycoproteins.
Full text
PDF

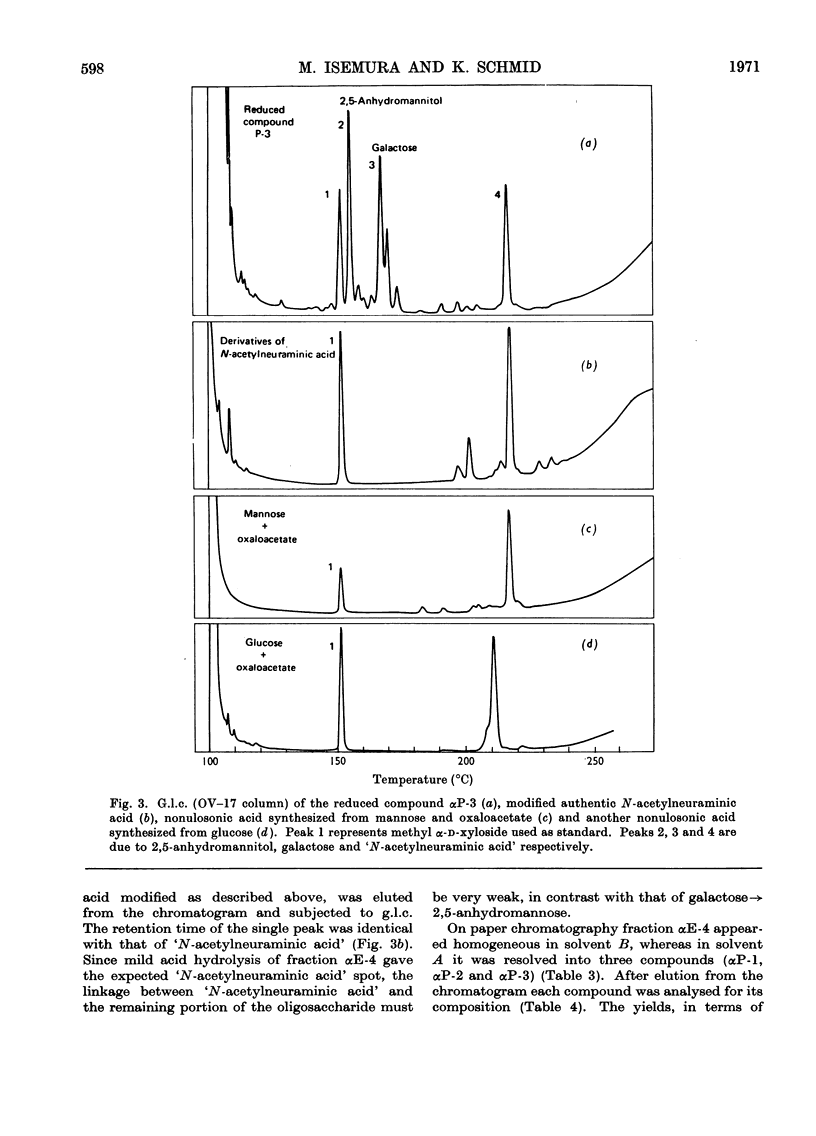
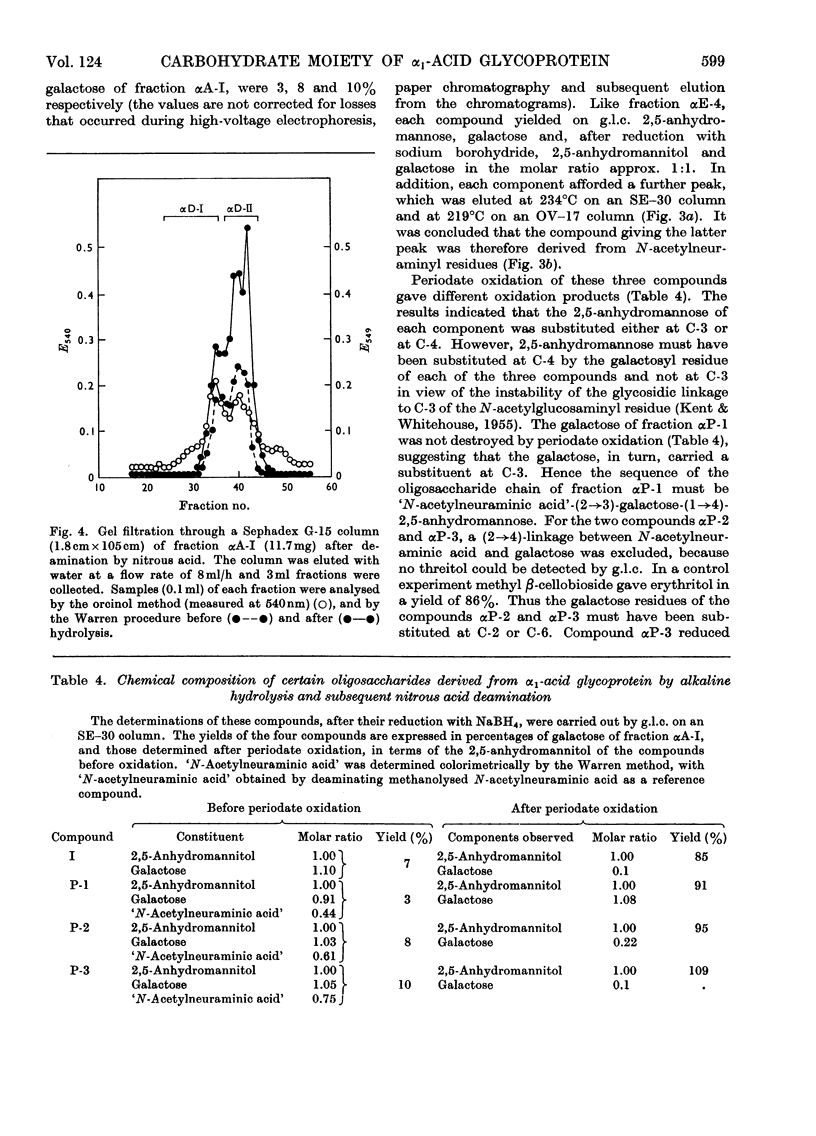
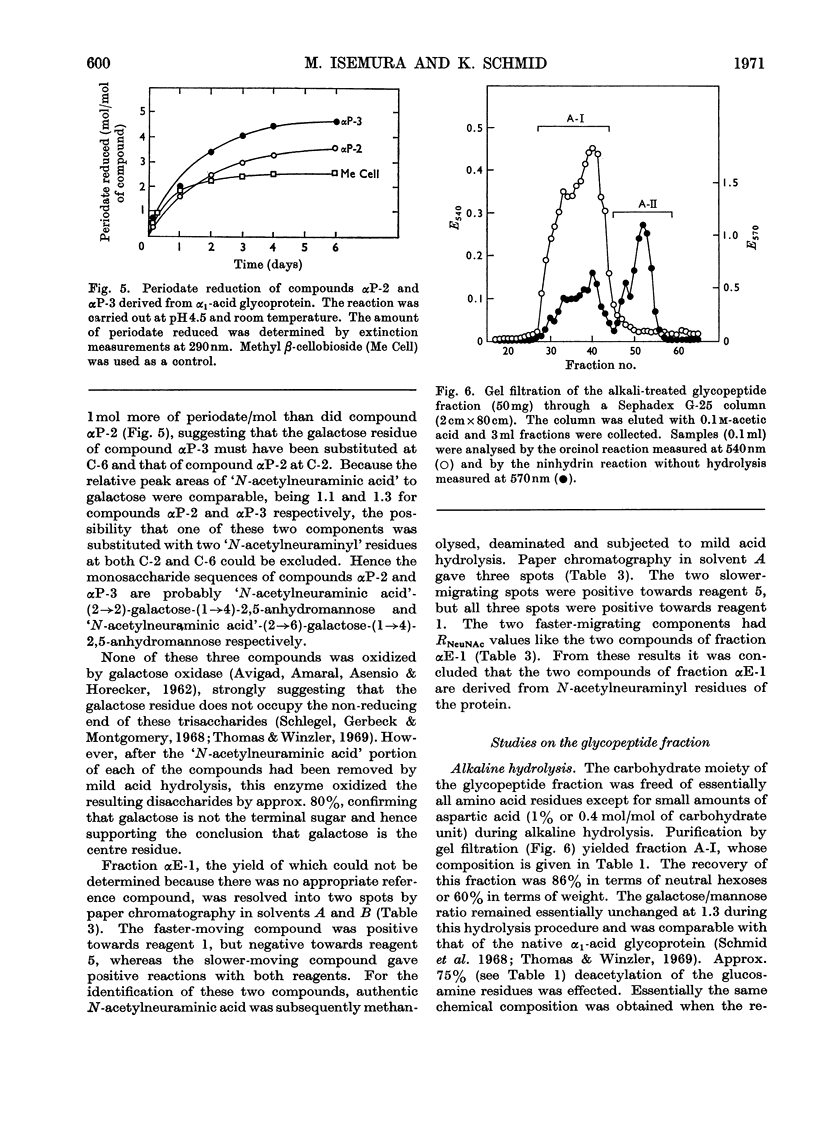



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVIGAD G., AMARAL D., ASENSIO C., HORECKER B. L. The D-galactose oxidase of Polyporus circinatus. J Biol Chem. 1962 Sep;237:2736–2743. [PubMed] [Google Scholar]
- BURGI W., SCHMID K. Preparation and properties of Zn-alpha 2-glycoprotein of normal human plasma. J Biol Chem. 1961 Apr;236:1066–1074. [PubMed] [Google Scholar]
- DISCHE Z., BORENFREUND E. A spectrophotometric method for the microdetermination of hexosamines. J Biol Chem. 1950 Jun;184(2):517–522. [PubMed] [Google Scholar]
- DISTLER J. J., MERRICK J. M., ROSEMAN S. Glucosamine metabolism. III. Preparation and N-acetylation of crystalline D-glucosamine- and D-galactosamine-6-phosphoric acids. J Biol Chem. 1958 Jan;230(1):497–509. [PubMed] [Google Scholar]
- GIBBONS R. A. THE SENSITIVITY OF THE NEURAMINOSIDIC LINKAGE IN MUCOSUBSTANCES TOWARDS ACID AND TOWARDS NEURAMINIDASE. Biochem J. 1963 Nov;89:380–391. doi: 10.1042/bj0890380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES R. C., JEANLOZ R. W. THE EXTRACELLULAR GLYCOSIDASES OF DIPLOCOCCUS PNEUMONIAE. I. PURIFICATION AND PROPERTIES OF A NEURAMINIDASE AND A BETA-GALACTOSIDASE. ACTION ON THE ALPHA-1-ACID GLYCOPROTEIN OF HUMAN PLASMA. Biochemistry. 1964 Oct;3:1535–1543. doi: 10.1021/bi00898a025. [DOI] [PubMed] [Google Scholar]
- Hershberger C., Davis M., Binkley S. B. Chemistry and metabolism of 3-deoxy-D-mannoctulosonic acid. II. Practical synthesis and stability. J Biol Chem. 1968 Apr 10;243(7):1585–1588. [PubMed] [Google Scholar]
- IKENAKA T. STUDIES ON THE MECHANISM OF THE ENZYMATIC ACTION OF TAKA-AMYLASE-A. ISOLATION AND IDENTIFICATION OF O-ALPHA-GLUCOPYRANOSYL-(1-4)-PHENYL-6-O-ACETYL-ALPHA-DGLUCOPYRANOSIDE. J Biochem. 1963 Oct;54:328–333. doi: 10.1093/oxfordjournals.jbchem.a127794. [DOI] [PubMed] [Google Scholar]
- Labat J., Schmid K. Neuraminidase-resistant sialyl residues of alpha 1-acid glycoprotein. Experientia. 1969;25(7):701–701. doi: 10.1007/BF01897570. [DOI] [PubMed] [Google Scholar]
- Paerels G. B., Schut J. The mechanism of the periodate-thiobarbituric acid reaction of sialic acids. Biochem J. 1965 Sep;96(3):787–792. doi: 10.1042/bj0960787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K., Okuyama T., Kaufmann H. Isolation and chemical composition of the human plasma alpha-1-acid glycoprotein variants. Biochim Biophys Acta. 1968 Apr 9;154(3):565–572. doi: 10.1016/0005-2795(68)90017-2. [DOI] [PubMed] [Google Scholar]
- Schmid K., Polis A., Hunziker K., Fricke R., Yayoshi M. Partial characterization of the sialic acid-free forms of alpha-1-acid glycoprotein from human plasma. Biochem J. 1967 Aug;104(2):361–368. doi: 10.1042/bj1040361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Thomas D. B., Winzler R. J. Structural studies on human erythrocyte glycoproteins. Alkali-labile oligosaccharides. J Biol Chem. 1969 Nov 10;244(21):5943–5946. [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- WEYGAND F., RINNO H. Vereinfachtes Verfahren zur Isolierung von Neuraminsäure als Methoxy-neuraminsäure mit Hilfe von Ionenaustauschern. Hoppe Seylers Z Physiol Chem. 1957 Feb 5;306(4-6):173–176. [PubMed] [Google Scholar]
- WHISTLER R. L., BEMILLER J. N. Alkaline degradation of polysaccharides. Adv Carbohydr Chem. 1958;13:289–329. doi: 10.1016/s0096-5332(08)60359-8. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Ikenaka T., Matsushima Y. The use of gas-liquid chromatography in the analysis of Smith degradation products from oligosaccharides. J Biochem. 1968 Apr;63(4):553–554. doi: 10.1093/oxfordjournals.jbchem.a128810. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Yamashina I. Isolation and analysis of glycopeptides from polymorphic variants of alpha 1-acid glycoprotein of human plasma. J Biochem. 1969 Aug;66(2):213–223. doi: 10.1093/oxfordjournals.jbchem.a129137. [DOI] [PubMed] [Google Scholar]
- Yosizawa Z., Sato T., Schmid K. Hydrazinolysis of alpha-1-acid glycoprotein. Biochim Biophys Acta. 1966 Jun 29;121(2):417–420. doi: 10.1016/0304-4165(66)90134-6. [DOI] [PubMed] [Google Scholar]
- Yosizawa Z., Sato T., Schmid K. Hydrazinolysis of alpha-1-acid glycoprotein. Biochim Biophys Acta. 1966 Jun 29;121(2):417–420. doi: 10.1016/0304-4165(66)90134-6. [DOI] [PubMed] [Google Scholar]


