Abstract
1. The release of growth hormone from isolated fragments of rat anterior pituitary tissue incubated in vitro was studied by employing a double-antibody radioimmunoassay. 2. In the absence of added stimuli, two phases of hormone release could be distinguished, an early phase of 2h duration and a subsequent late phase. In the early phase, hormone release was rapid but could be significantly decreased by calcium depletion and by 2,4-dinitrophenol whereas the rate of release in the late phase was uninfluenced by these incubation conditions. These results have been interpreted as indicating the existence of a secretory component in the early phase of release. 3. In subsequent experiments, the effects of various agents on the rate of hormone output during the late phase of incubation were investigated. Hormone release was increased by theophylline and by dibutyryl cyclic AMP (N6-2′-O-dibutyryl-adenosine 3′:5′-cyclic monophosphate), the response to both of these agents being related to the concentration of the stimulant employed. 4. The stimulation of growth hormone output by theophylline was significantly decreased by calcium deprivation and by 2,4-dinitrophenol. The response to dibutyryl cyclic AMP was diminished by 2,4-dinitrophenol, iodoacetate and 2-deoxyglucose but not by malonate or colchicine. 5. Arginine, β-hydroxybutyrate, albumin-bound palmitate and variation in the glucose concentration of the incubation medium over a wide range were without any statistically significant effect on the rate of hormone release from either control pituitary fragments or those subject to secretory stimulation by dibutyryl cyclic AMP. 6. It is suggested that the regulation of growth hormone secretion is mediated by cyclic AMP (adenosine 3′:5′-cyclic monophosphate). The secretion observed in response to cyclic AMP requires the presence of ionized calcium and a source of metabolic energy but is independent of pituitary protein synthesis de novo. The integrity of the glycolytic pathway of glucose metabolism appears to be essential for cyclic AMP-stimulated growth hormone secretion to occur.
Full text
PDF



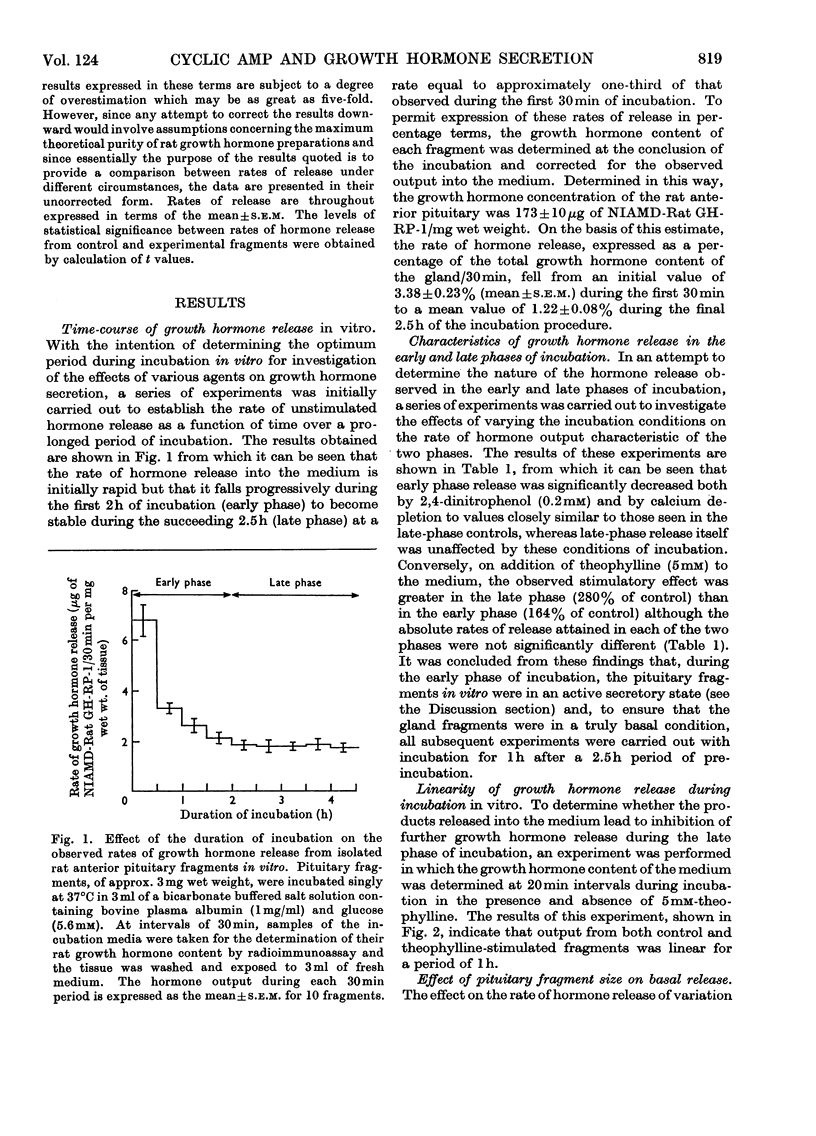






Selected References
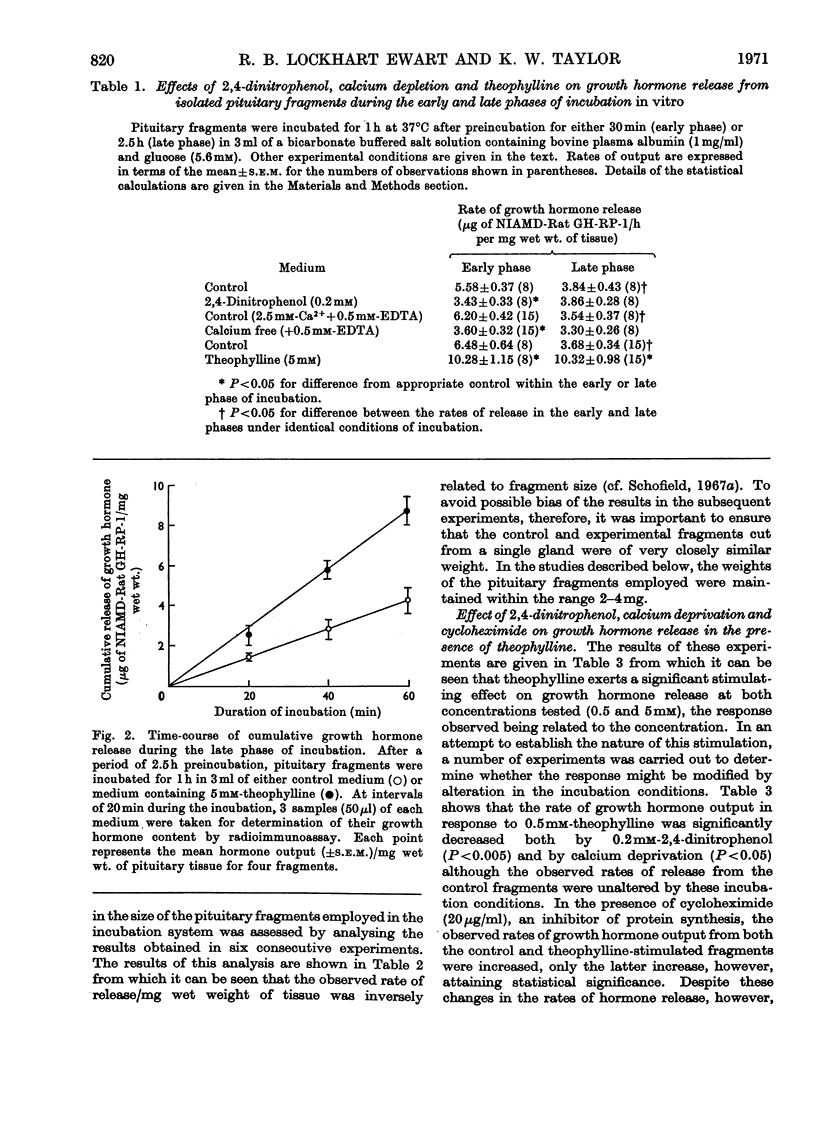
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams R. L., Parker M. L., Blanco S., Reichlin S., Daughaday W. H. Hypothalamic regulation of growth hormone secretion. Endocrinology. 1966 Mar;78(3):605–613. doi: 10.1210/endo-78-3-605. [DOI] [PubMed] [Google Scholar]
- BROWN J. B., BULBROOK R. D., GREENWOOD F. C. An evaluation of a chemical method for the estimation of oestriol, oestrone and oestradiol-17 beta in human urine. J Endocrinol. 1957 Nov;16(1):41–48. doi: 10.1677/joe.0.0160041. [DOI] [PubMed] [Google Scholar]
- Birge C. A., Peake G. R., Mariz I. K., Daughaday W. H. Effects of cortisol and diethylstilbestrol on growth hormone release by rat pituitary in vitro. Proc Soc Exp Biol Med. 1967 Nov;126(2):342–345. doi: 10.3181/00379727-126-32440. [DOI] [PubMed] [Google Scholar]
- DEUBEN R. R., MEITES J. STIMULATION OF PITUITARY GROWTH HORMONE RELEASE BY A HYPOTHALAMIC EXTRACT IN VITRO. Endocrinology. 1964 Mar;74:408–414. doi: 10.1210/endo-74-3-408. [DOI] [PubMed] [Google Scholar]
- Fawcett D. W., Long J. A., Jones A. L. The ultrastructure of endocrine glands. Recent Prog Horm Res. 1969;25:315–380. doi: 10.1016/b978-0-12-571125-8.50010-7. [DOI] [PubMed] [Google Scholar]
- Greenwood F. C., Landon J. Growth hormone secretion in response to stress in man. Nature. 1966 Apr 30;210(5035):540–541. doi: 10.1038/210540a0. [DOI] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Henion W. F., Sutherland E. W., Posternak T. Effects of derivatives of adenosine 3',5'-phosphate on liver slices and intact animals. Biochim Biophys Acta. 1967 Oct 9;148(1):106–113. doi: 10.1016/0304-4165(67)90284-x. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Howell S. L., Young D. A., Fink C. J. New hypothesis of insulin secretion. Nature. 1968 Sep 14;219(5159):1177–1179. doi: 10.1038/2191177a0. [DOI] [PubMed] [Google Scholar]
- Merimee T. J., Lillicrap D. A., Rabinowitz D. Effect of arginine on serum-levels of human growth-hormone. Lancet. 1965 Oct 2;2(7414):668–670. doi: 10.1016/s0140-6736(65)90399-5. [DOI] [PubMed] [Google Scholar]
- Müller E. E., Pecile A., Naimzada M. K., Ferrario G. The involvement of cyclic 3',5'-adenosine monophosphate in the growth hormone release mechanism (s). Experientia. 1969;25(7):750–751. doi: 10.1007/BF01897605. [DOI] [PubMed] [Google Scholar]
- ROTH J., GLICK S. M., YALOW R. S., BERSON S. A. Secretion of human growth hormone: physiologic and experimental modification. Metabolism. 1963 Jul;12:577–579. [PubMed] [Google Scholar]
- Randle P. J., Newsholme E. A., Garland P. B. Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):652–665. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHALLY A. V., STEELMAN S. L., BOWERS C. Y. EFFECT OF HYPOTHALAMIC EXTRACTS ON RELEASE OF GROWTH HORMONE IN VITRO. Proc Soc Exp Biol Med. 1965 May;119:208–212. doi: 10.3181/00379727-119-30138. [DOI] [PubMed] [Google Scholar]
- Samli M. H., Geschwind I. I. Some effects of energy-transfer inhibitors and of Ca++-free or K+-enhanced media on the release of luteinizing hormone (LH) from the rat pituitary gland in vitro. Endocrinology. 1968 Feb;82(2):225–231. doi: 10.1210/endo-82-2-225. [DOI] [PubMed] [Google Scholar]
- Schofield J. G. Measurement of growth hormone released by ox anterior-pituitary slices in vitro. Biochem J. 1967 May;103(2):331–341. doi: 10.1042/bj1030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield J. G. Role of cyclic 3',5'-adenosine monophosphate in the release of growth hormone in vitro. Nature. 1967 Sep 23;215(5108):1382–1383. doi: 10.1038/2151382b0. [DOI] [PubMed] [Google Scholar]
- Schofield J. G., Stead Margaret. ATP, calcium uptake and growth hormone release. FEBS Lett. 1971 Mar 5;13(3):149–151. doi: 10.1016/0014-5793(71)80222-3. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Peake G. T., Utiger R. D., Karl I. E., Kipnis D. M. Hypothalamic stimulation of growth hormone and thyrotropin lease in vitro and pituitary 3'5'-adenosine cyclic monophosphate. Endocrinology. 1970 Jun;86(6):1354–1360. doi: 10.1210/endo-86-6-1354. [DOI] [PubMed] [Google Scholar]
- Wilber J. F., Utiger R. D. In vitro studies on mechanism of action of thyrotropin releasing factor. Proc Soc Exp Biol Med. 1968 Feb;127(2):488–490. doi: 10.3181/00379727-127-32722. [DOI] [PubMed] [Google Scholar]
- Williams J. A., Wolff J. Possible role of microtubules in thyroid secretion. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1901–1908. doi: 10.1073/pnas.67.4.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]


