Abstract
Cytokine signaling by the Jak–STAT pathway is subject to complex negative regulation that limits the amplitude and duration of signal transduction. Inhibition of signaling also mediates negative crosstalk, whereby factors with opposing biological activities crossinhibit each other's function. Here, we investigated a rapidly inducible mechanism that inhibited Jak–STAT activation by IFN-α, a cytokine that is important for antiviral responses, growth control, and modulation of immune responses. IFN-α-induced signaling and gene activation were inhibited by ligation of Fc receptors and Toll-like receptors 7 and 8 in a PKCβ-dependent manner. Neither PKCβ nor PKCδ influenced responses of cells treated with IFN-α alone. Inhibition of IFN-α signaling correlated with suppression of IFN-α-dependent antiviral responses. PKC-mediated inhibition did not require de novo gene expression but involved the recruitment of PKCβ to the IFN-α receptor and interaction with protein tyrosine phosphatase SHP-2, resulting in augmented phosphatase activity. PKC-mediated inhibition of IFN-α signaling was abolished in SHP-2-deficient cells, demonstrating a pivotal role for SHP-2 in this inhibitory pathway. Together, our data describe a rapidly inducible, direct mechanism of inhibition of Jak–STAT signaling mediated by a PKCβ–SHP-2 signaling pathway.
Keywords: interferon, macrophage, Stat
Type I IFNs (IFNs α/β) are pleiotropic cytokines with potent antiviral, growth-inhibitory, antitumor, and immunomodulatory properties (1–3). Type I IFNs elicit cellular responses by binding to a heterodimeric receptor termed IFNAR that consists of IFNAR1 and IFNAR2 subunits. Ligation of IFNAR results in activation of the receptor-associated Janus tyrosine kinases, Jak1 and Tyk2, which in turn leads to tyrosine phosphorylation and activation of latent transcription factors termed STATs (4). Activated STATs then form homodimers or heterodimers and translocate into the nucleus, bind to conserved promoter sequences, and induce the transcription of IFN-responsive genes. IFN-α/β primarily activates Stat1, Stat2, and Stat3 in most types of cells and can also activate Stat4, Stat5, and Stat6 in a cell type-specific manner (2). With IRF9, Stat1:Stat2 heterodimers form the IFN-stimulated gene factor 3, which binds to cis-acting DNA sequences termed IFN-stimulated response elements. Stat1:Stat1 and Stat3:Stat3 homodimers and Stat1:Stat3 heterodimers formed in response to IFN-α/β stimulation bind to IFN-γ-activated sequence elements. The activation of multiple STATs and other related signaling pathways by type I IFNs may explain the pleiotropic nature of this cytokine and its transcriptional activation of a plethora of genes that mediate the induction of biologic responses.
Prominent effects of type I IFNs are their antiviral and antiproliferative actions (1, 2). Thus, IFN-α/β has been used as a therapeutic reagent in the treatment of a variety of diseases, such as cancer and viral infection (5–7). However, resistance to the effects of IFN-α/β is an important clinical problem. A significant number of patients have been reported not to respond to IFN even at the time of initial therapy, and many patients become resistant after long-term treatment (8, 9). IFN-α signaling and function can be suppressed by several mechanisms, including suppression that occurs during antibody-mediated enhancement of infections of macrophages by single-stranded RNA (ssRNA) viruses, which is presumably mediated by Toll-like receptors (TLRs) and Fc receptors (10). Further investigation of mechanisms of inhibition of IFN-α/β signaling will yield insights into viral pathogenesis and may facilitate the development of therapeutic approaches that overcome IFN resistance.
Many mechanisms that suppress cytokine and IFN signaling by means of the Jak–STAT pathway have been described (11–13), including (i) inhibition of receptor expression or targeting receptors for proteolytic destruction by suppressors of cytokine signaling (SOCS), (ii) inhibition of Jak catalytic activity by SOCS, (iii) dephosphorylation of receptor or Jak tyrosine residues mediated by the SH2-containing phosphatases SHP-1 and SHP-2 and by CD45, (iv) dephosphorylation of STATs in the nucleus, and (v) inhibition of STAT DNA binding by protein inhibitors of activated STATs (14). Our laboratory and others have described rapidly activated pathways that inhibit signaling by IL-2, IL-4, IL-6, and IL-10 by mechanisms that depend on activation of extracellular signal-regulated kinases (ERKs), p38, or PKC (15–24). Rapid inhibition of cytokine signaling mediated by these kinases mostly occurs at the level of the receptor or associated signaling proteins by means of posttranslational modification, which is independent of de novo protein synthesis. Thus, ERKs, p38, and PKC can inhibit cytokine signaling independently of the induction of SOCS expression. However, the molecular targets of mitogen-activated protein kinases (MAPKs) and PKC and the precise mechanism by which they inhibit cytokine signaling have not been delineated.
In this report, we demonstrate that IFN-α signaling is inhibited by a rapidly inducible, direct inhibitory pathway that requires both PKCβ and SHP-2. We propose a model whereby, after activation, PKCβ is recruited to IFNAR and inhibits IFN-α signaling by activating the catalytic activity of IFNAR-associated SHP-2. Therefore, our results further delineate a mechanism of the negative regulation of Jak–STAT signaling.
Materials and Methods
Cell Culture and Reagents. Cell lines were purchased from American Type Culture Collection and cultured in RPMI medium 1860 with 10% FBS. NIH 3T3 cells, immortalized SHP-2-deficient fibroblasts, and SHP-2-deficient cells reconstituted with SHP-2 were maintained in DMEM with 10% FBS as described in ref. 25. Transgenic mice carrying the SHP-2 floxed allele (Shp-2fl/fl) were crossed to a Cre transgenic mouse line in which the Cre recombinase expression is under the control of the M lysozyme promoter to generate Shp-2fl/fl;LysMCre mice, in which the Shp-2 gene is deleted in myeloid cells (unpublished data). Primary human monocytes and murine bone marrow-derived macrophages were obtained as described in refs. 15 and 16. Phorbol 12-myristate 13-acetate (PMA) was from Sigma-Aldrich, IFN-α was from Peptotech (Rocky Hill, NJ), and antibodies against Stat1, Stat2, Stat3, and SHP-2 were from Cell Signaling Technology (Beverly, MA) or BD Transduction Laboratories. GF109203X, LY 294002, SB203580, PD98059, and actinomycin D were purchased from Calbiochem. Heat-aggregated IgG were prepared by incubation of IgG (Sigma-Aldrich) at 63°C for 20 min.
Immunoblotting, EMSA, and Immunoprecipitation. Whole-cell extract preparation, immunoblotting, and EMSA were performed as described in ref. 16. For immunoprecipitations, cells were lysed in lysis buffer containing 20 mM Tris·HCl (pH 6.6), 1% Brij58 or Triton X-100, 150 mM NaCl, 1 mM EDTA, 10% glycerol, 1.0 mM sodium fluoride, 1.0 mM sodium orthovanadate, 1.0 mM PMSF, 0.5 μg/ml leupeptin, and 5.0 μg/ml trypsin inhibitor. Cell lysates were incubated overnight at 4°C with immunoprecipitating antibodies.
In Vitro Phosphatase Assay. Phosphatase assays were carried out as described in ref. 26 with anti-SHP-2 antibodies. The release of free phosphate was measured by using malachite green (Upstate Biotechnology, Lake Placid, NY) according to the manufacturer's instructions.
Immunofluorescence and Confocal Microscopy. Cells were fixed and permeabilized with cold 100% methanol for 5 min at room temperature or with 0.1% saponin/0.5% gelatin in PBS for 10 min at room temperature and stained with primary antibodies. Alexa Fluor 488- or Alexa Fluor 594-conjugated secondary antibodies (Molecular Probes) were used. Slides were visualized by using a confocal fluorescence microscope (model LSM510, Zeiss). Colocalization analyses were performed by using metamorph software (Universal Imaging, Downingtown, PA).
Cell Surface Biotinylation and Membrane Fractionation. The cell surface biotinylation assay was performed as described in ref. 27. Membrane fractionation was carried out as described in ref. 28.
Results
Inhibition of IFN-α-Induced Jak–STAT Signaling by PKC. Based on the suggestion that Fc receptors and TLRs may contribute to the suppression of the earliest antiviral effects of IFN-α/β(10), we investigated the effects of activating macrophage Fcγ receptors (FcγRs) or TLRs on IFN-α signaling. IFN-α induced tyrosine phosphorylation of both Stat1 and Stat3, as expected (Fig. 1). Crosslinking of FcγRs, or incubation with R848, a synthetic ligand that activates TLR7 and TLR8 and is generally used as a mimic of the effects of viral ssRNA (29, 30), before addition of IFN-α inhibited IFN-α-induced tyrosine phosphorylation of Stat1 and Stat3 (Fig. 1 A and B). Given that PKC is involved in FcγR signaling (31) and also in FcγR-mediated inhibition of Jak–STAT signaling (15, 19–21), we investigated whether the inhibition of IFN-α signaling depended on PKC. FcγR- and R848-mediated inhibition of IFN-α signaling was abolished when the selective PKC inhibitor GF109203X was added, suggesting that PKC was required for inhibition of IFN-α signaling by both stimuli (Fig. 1 A and B). Experiments using macrophages deficient in specific PKC isozymes (32) demonstrated that inhibition of IFN-α signaling by both FcγRs and R848 depended on PKCβ but not on PKCδ (Fig. 1 C and D).
Fig. 1.
Inhibition of IFN-α-induced Jak–STAT signaling by FcγRs and R848 is mediated by PKC. (A)FcγRs on primary human macrophages were crosslinked by using plate-bound IgG for 1 h with or without GF109203X pretreatment and stimulated for 10 min with IFN-α (1,000 units/ml), and STAT activation was measured by using immunoblotting. (B) Macrophages were treated with R848 (1 μg/ml) for 1 h with or without GF109203X pretreatment and then stimulated with IFN-α. (C and D) Peritoneal macrophages from PKCβ- or PKCδ-deficient or genetically matched control mice were used.
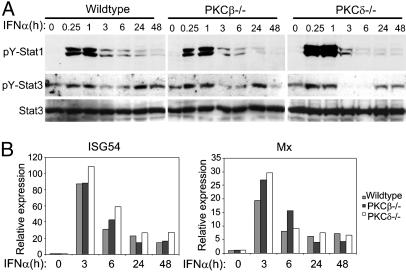
We investigated whether PKCβ regulated IFN-α signaling independently of preactivation of FcγRs or TLR7/8 by comparing the amplitude and kinetics of Stat1 and Stat3 tyrosine phosphorylation in wild-type and PKCβ- and PKCδ-deficient macrophages. Activation of STATs by IFN-α was nearly identical in wild-type and PKCβ-deficient macrophages and was modestly increased at early time points in PKCδ-deficient macrophages (Fig. 2A). Consistent with similar patterns of signal transduction, the activation of Mx and ISG54 mRNA expression by IFN-α was comparable in wild-type and PKCβ- and PKCδ-deficient macrophages (Fig. 2B). The results, taken together, indicate that PKCβ mediates modulation of IFN-α signaling by other ligands, whereas PKCδ appears to play a modest role in regulating the amplitude of the IFN-α response in the absence of other ligands. Coordinate regulation of tyrosine kinase signaling by two different PKC isozymes acting by different mechanisms has been previously reported (33).
Fig. 2.
Effects of PKCβ and PKCδ on IFN-α-induced signaling and gene expression. (A) Bone marrow-derived macrophages from PKCβ- or PKCδ-deficient or genetically matched control mice were stimulated with IFN-α (1,000 units/ml) for the indicated times, and cells lysates were analyzed by immunoblotting. (B) Mx and ISG54 mRNA levels were measured by using real-time PCR and normalized relative to GAPDH mRNA.
We used the direct PKC activator PMA to determine whether activation of PKC was sufficient to inhibit IFN-α-induced STAT activation. Pretreatment of primary human macrophages with PMA inhibited IFN-α-induced STAT tyrosine phosphorylation (Fig. 3A), DNA binding (Fig. 3B), and nuclear translocation (Fig. 3C). Inhibition of IFN-α-induced STAT activation was not limited to primary macrophages and was also observed in THP-1, RAW267.4, NIH 3T3, and COS cells (data not shown). Activation of PKC resulted in the rapid inhibition of IFN-α signaling; a 5-min preincubation with PMA was sufficient to inhibit IFN-α-induced STAT activation (Fig. 3 A and B). Moreover, PMA-induced inhibition of IFN-α signaling was reversed by the PKC inhibitor GF109203X but not by inhibitors of several other kinases, including MAPKs that are activated downstream of PKC, or when de novo gene expression was blocked by using actinomycin D (Fig. 3D). Similar to FcγR ligation and R848 (Fig. 1), inhibition of IFN-α signaling by PMA depended on PKCβ(Fig. 3E). Taken together, these results indicate that PKCβ rapidly inhibits IFN-α signaling and suggest that the mechanism of inhibition is direct and does not require activation of downstream kinases or de novo expression of inhibitors.
Fig. 3.
PKC activation is sufficient for inhibition of IFN-α signaling. (A and B) Human macrophages were incubated with 100 ng/ml PMA for the indicated times, stimulated with IFN-α (1,000 units/ml) for 10 min, and whole-cell extracts were analyzed by immunoblotting (A) or EMSA (B). An autoradiograph representative of three independent experiments is shown in A and B. (C) Macrophages were analyzed by using immunofluorescence microscopy after treatment with IFN-α (1,000 units/ml) for 30 min with or without a 30-min pretreatment with PMA (100 ng/ml). (D) Macrophages were incubated with 4 μM GF109203X, 10 μM LY 294002, 10 μM SB203580, 50 μM PD980591, and 5 μg/ml actinomycin D for 30 min to inhibit PKC, phosphatidylinositol 3-kinase, p38 kinase, extracellular signal-regulated kinase/MAPK, and RNA synthesis, respectively; PMA (100 ng/ml) was then added 30 min before the cells were stimulated with IFN-α. Cell extracts were analyzed by EMSA and immunoblotting. (E) Bone marrow-derived macrophages from PKCβ-deficient or genetically matched control mice were stimulated with IFN-α A/D with or without 1 h of pretreatment with PMA (100 ng/ml).
PKC Inhibits IFN-α-Mediated Gene Activation and Antiviral Responses. We tested the effects of PMA, FcγR crosslinking, and R848 on IFN-α-induced activation of gene expression. PMA and FcγR crosslinking did not increase the expression of oligoadenylate synthetase (OAS), Mx, Cig5, or ISG54 mRNA but effectively suppressed IFN-α induction of these genes (Fig. 4Aa). In contrast, R848 induced expression of OAS, Mx, Cig5, and ISG54 mRNA, consistent with the induction of endogenous type I IFNs by this compound that has antiviral properties (34). Interestingly, R848 prevented the stronger activation of these genes by exogenous IFN-α. These results are consistent with a feedback inhibition loop whereby TLRs7/8 limit the autocrine activity of type I IFNs produced in response to TLR7/8 ligation. In contrast to wild-type murine macrophages, inhibition of IFN-α-induced gene expression by PMA, FcγR crosslinking, or R848 was not observed in PKCβ-deficient macrophages (Fig. 4Ab), further supporting a role for PKCβ in FcγR and R848 inhibition of type I IFN signaling. Inhibition of the induction of the OAS and Mx genes that have antiviral function suggested that inhibition of IFN-α signaling would lead to a diminished IFN-α-mediated antiviral response, which depends on activation of the Jak–STAT signaling pathway (3, 4). We examined whether PKC activation affected IFN-α-mediated antiviral responses by using a standard cytopathic effect assay. In this assay, 2fTGH cell monolayers treated with a maximally protective dose of IFN-α with or without a 60-min PMA pretreatment were infected with serial dilutions of encephalomyocarditis virus. Protection by IFN-α was quantitated by comparing the virus concentrations required to generate 50% of cell killing. As expected, IFN-α induced >1,000-fold antiviral protection (Fig. 4B), whereas this IFN-α-mediated antiviral effect was almost completely abrogated when cells were pretreated with PMA (Fig. 4 B and C). Thus, inhibition of IFN-α signaling correlated with the inhibition of an important physiological function of IFN-α.
Fig. 4.
IFN-α-mediated gene activation and antiviral responses are suppressed by PKC. (A) Primary human macrophages (a) and control or PKCβ-deficient murine peritoneal macrophages (b) were preincubated with PMA (100 ng/ml), plate-bound IgG, or R848 (1 μg/ml) for 1 h and stimulated with IFN-α (50 ng/ml) for 3 h, and mRNA levels were measured by using real-time PCR and normalized relative to GAPDH mRNA. (B) 2fTGH cells were treated with IFN-α for 6 h with or without a 1-h pretreatment with PMA. Serial dilutions of encephalomyocarditis virus were added, and, after 24 h, the viable cells were visualized by methylene blue staining. (C) Results from B were quantitated by measuring the absorbance of methylene blue at 630 nm, and the IFN-α protection efficiency was determined by comparing the virus concentrations required to kill 50% of cells.
PKC Inhibits a Proximal Step in IFN-α Signaling Upstream of STATs. PKC can inhibit cellular responses to extracellular ligands by down-regulating receptor expression (15, 35). Therefore, we investigated the effects of PKC activation on cell surface expression of IFNAR. In the first approach, we specifically precipitated cell surface proteins by using cell surface biotinylation followed by avidin-mediated precipitation and used immunoblotting to measure cell surface expression of IFNAR. PMA treatment did not affect cell surface receptor expression of IFNAR1 or IFNAR2 (Fig. 5A), indicating that PMA did not inhibit IFN-α signaling by modulating IFNAR expression at the cell surface. In support of this finding, confocal microscopy showed little change in cell surface IFNAR expression after PMA treatment (Fig. 5B). However, PMA induced a translocation of PKCβ to the plasma membrane, where PKCβ colocalized with the IFNAR (Fig. 5 B and C). R848 and FcγR ligation also induced translocation of PKCβ to membranes (Fig. 5C), thus confirming activation of PKCβ by these ligands in our system. TLRs have been previously shown to activate PKC (36), and FcγRs have been shown to activate PKCβ (31). These results, together with PMA-induced coimmunoprecipitation of IFNAR and PKCβ (see below), suggested that PKCβ inhibits IFN-α signaling at the level of the IFNAR and thus inhibits a proximal step in IFN-α signal transduction. This notion was tested by measuring the effects of PMA on the activation of IFNAR-associated Jaks. IFN-α rapidly induced the phosphorylation of both Jak1 and Tyk2, whereas pretreatment with PMA suppressed Jak1 and Tyk2 phosphorylation (Fig. 5D). These results suggested that PKC inhibited the initiation of the IFN-α-induced Jak–STAT signaling cascade, possibly by phosphorylating IFNAR-associated proteins.
Fig. 5.
PKC inhibits IFN-α-induced activation of Jak1 and Tyk2 without altering cell surface IFNAR expression. (A) Biotinylated THP-1 cell surface proteins were precipitated with avidin-agarose beads and analyzed by immunoblotting with IFNAR1 and 2 antibodies. Blots were subsequently stripped and reprobed with an anti-CD45 mAb as a loading control. (B) PMA-induced PKCβ translocation to plasma membranes and colocalization with IFNAR. Permeabilized THP-1 cells were incubated with IFNAR2 and PKCβ antibodies and analyzed by confocal microscopy. (C) Membrane fractions from macrophages stimulated with PMA, IgG, or R848 were analyzed by using immunoblotting; Coomassie blue staining of membrane fractions was used for loading controls. (D) THP-1 cells were stimulated with IFN-α with or without pretreatment with PMA (100 ng/ml), and Jak1 and Jak2 immunoprecipitates were analyzed by immunoblotting. Representative results from three experiments are shown.
SHP-2 Is Essential for PKC-Mediated Inhibition of IFN-α Signaling. IFNAR has been reported to be associated with receptor for activated C-kinase 1 (RACK1) (37), which is a specific intracellular receptor for activated PKCβ. Therefore, we investigated whether PKCβ is recruited to IFNAR2, which is preassociated with RACK1, Jak1, and SHP-2 (3, 37–39). To examine the physical association of PKCβ with IFNAR, we performed coimmunoprecipitation assays. PMA treatment resulted in an increase in the association of PKCβ with IFNAR2 that was highly reproducible in a stimulus- and time-dependent manner (Fig. 6A), supporting the notion that the recruitment of PKCβ to IFNAR mediates inhibition of signaling. Among potential mediators of inhibition of IFN-α signaling, we considered SHP-2, which is a phosphorylation target for PKC (40), constitutively associates with IFNAR (38, 39), and regulates IFN-α signaling (38, 39). Coimmunoprecipitation assays showed that PMA induced the association of PKCβ with SHP-2 (Fig. 6B), which was also associated with Jak1 and RACK1, likely in the IFNAR signaling complex (Fig. 6 A and B). These results suggested that activated PKCβ could phosphorylate and thereby activate SHP-2. We then tested the effects of IFN-α and PMA on SHP-2 phosphatase activity. SHP-2 was immunoprecipitated from cells that had been treated with IFN-α with or without PMA pretreatment, and SHP-2 catalytic activity was analyzed by an in vitro phosphatase assay. IFN-α alone induced an increase in SHP-2 activity (Fig. 6C), consistent with a constitutive role for SHP-2 in limiting IFNAR signaling (39, 41, 42). PMA pretreatment resulted in a superinduction of SHP-2 activity, suggesting that PKC-dependent activation of SHP-2 contributes to PKC-dependent inhibition of IFN-α signaling. Furthermore, the PMA-induced increase in SHP-2 activity was inhibited by the PKC inhibitor GF109203X (Fig. 6D). These results suggested a role for SHP-2 in mediating PKC-induced inhibition of IFN-α signaling. To more definitively address the role of SHP-2 in PKC-induced inhibition of IFN-α signaling, we compared the effects of FcγR crosslinking and R848 on IFN-α signaling in macrophages derived from mice containing floxed shp-2 alleles and mice in which the shp-2 gene had been specifically deleted in myeloid lineage cells. Inhibition of IFN-α signaling by both FcγR ligation and R848 was partially reversed in SHP-2-deficient cells (Fig. 6E), indicating a role for SHP-2 in inhibition of signaling. Because macrophages express SHP-1 that can potentially compensate for SHP-2 function, we also tested SHP-2-deficient fibroblasts that do not express SHP-1. Wild-type fibroblasts were sensitive to the inhibitory effect of PMA, whereas this inhibitory effect was abrogated in SHP-2-deficient cells (Fig. 6F). Moreover, reconstitution of SHP-2-deficient cells with wild-type SHP-2 restored the ability of PMA to inhibit IFN-α signaling (Fig. 6F). Taken together, these data indicate that SHP-2 is required for PKC-dependent inhibition of IFN-α signaling and suggest that a signal pathway involving PKCβ→SHP-2→Jak kinases modulates the amplitude of IFN-α signaling.
Fig. 6.
PKC-mediated inhibition of IFN-α signaling depends on SHP-2. (A) PMA-induced association of PKCβ with IFNAR2. Serum-starved THP-1 monocytic cells were treated with 100 ng/ml PMA for indicated time periods, and immunoprecipitates obtained by using IFNAR2 or preimmune antiserum (Pre.) were analyzed by immunoblotting. Data are representative of three experiments. (B) PMA-induced association of PKCβ with SHP-2. Serum-starved THP-1 monocytic cells were treated with 100 ng/ml PMA for 1 h or left untreated, and immunoprecipitates obtained by using SHP-2 or preimmune antiserum (Pre.) were analyzed by immunoblotting. (C and D) SHP-2 in vitro phosphatase assay. SHP-2 phosphatase activity was analyzed in SHP-2 immunoprecipitates from serumstarved THP-1 cells that had been treated with PMA and IFN-α. In D, 4 μM GF109203X was added 20 min before adding PMA. Representative results of three independent experiments are shown. (E) Macrophages derived from mice containing floxed shp-2 alleles (Shp-2fl/fl) and mice in which the shp-2 gene had been specifically deleted in myeloid lineage cells (Shp-2fl/fl;LysMCre) were pretreated with heat-aggregated mouse IgG or R848 and stimulated with IFN-α, and whole-cell extracts were analyzed by immunoblotting. (F) Serum-starved wild-type cells, SHP-2-deficient cells (Shp-2-/-), and SHP-2-deficent cells that had been reconstituted with SHP-2 (Shp-2+/+) were treated with 1,000 units/ml IFN-αA/D with or without 1 h of PMA pretreatment.
Discussion
Negative regulation of the cytokine-activated Jak–STAT signaling pathway is extremely complex, reflecting the need to fine-tune cytokine signaling and avoid potentially deleterious consequences of excessive cell activation. We and others have described a direct pathway that inhibits Jak–STAT signaling where inhibition is mediated by rapid modification of signaling molecules by serine/threonine kinases of the PKC or MAPK families (15–24). However, the molecular targets for these kinases have not been identified, and the mechanisms of inhibition have not been clarified. In this report, we have delineated a direct pathway that inhibits IFNAR signaling by means of PKCβ-mediated activation of SHP-2, which provides a mechanism for crosstalk between the Jak–STAT pathway and other signaling pathways that activate PKCβ.
Inhibition of Jak–STAT signaling mediated by de novo induction of SOCS protein expression is the most thoroughly characterized pathway for down-regulating Jak–STAT signaling. SOCS inhibit cytokine signaling by several mechanisms, including targeting receptors for proteolytic degradation, inhibiting Jak catalytic activity, and competing with STATs for receptor docking sites. Less is known about direct kinase-dependent signaling crosstalk and inhibition of STAT activation, but the emerging evidence suggests a similar complexity in that several kinases can inhibit different receptors by different mechanisms. Activation of the extracellular signal-regulated kinase and p38 MAPKs by factors such as IL-1 and TNF effectively inhibits IL-6 signaling by a mechanism that appears to involve recruitment of SHP-2 to IL-6 receptors (23, 43). In contrast, cytokines such as IFNs and IL-10 are resistant to inhibition by MAPKs (24) but are inhibited by PKC, which also contributes to the inhibition of IL-2 and IL-4 signaling in T cells (17, 22). Within the PKC family, PKCδ inhibits IL-10 signaling by inducing internalization of the IL-10 receptor (15); this study shows that PKCβ does not affect cell surface IFNAR expression but inhibits IFN-α signaling by activating IFNAR-associated SHP-2. Thus, MAPKs and PKC isozymes target different signaling molecules to inhibit signaling by different cytokines.
Our results demonstrate that physiological stimuli that induce PKCβ translocation to membranes, where PKCβ interacts with IFNAR, inhibit IFN-α signaling. Activated PKCβ is recruited to membranes through interaction with its intracellular receptor, RACK1. Therefore, PKCβ can potentially inhibit signaling by cytokine receptors that associate with RACK1. Although to date IFNAR is the only cytokine receptor with which RACK1 is known to associate, RACK1 has been shown to interact with Tyk2 (44). Thus, it is possible that PKCβ inhibits signaling by other Tyk2-associated receptors, such as members of the IFN-λ, IL-10, and IL-12 receptor families (45). However, because SHP-2 does not associate with receptors of the IL-10 and IL-12 receptor families, PKCβ would need to regulate the function of these receptors by phosphorylating other receptor-associated proteins or the receptors themselves. Given the precedents that PKCs can negatively regulate the catalytic activity of protein tyrosine kinases, including Jak2 (19), it is possible that PKCβ inhibits receptor-associated Jaks by direct phosphorylation.
IFN-α signaling is regulated during the course of an immune response (46), and our results suggest that immune complexes and Fc receptors can contribute to this regulation. Interestingly, IFN-α signaling was also suppressed by the guanosine analog R848 that activates TLRs 7 and 8 and is widely used as a mimic of ssRNA that is produced during viral infection and induces type I IFN production. This finding suggests that production of ssRNA during a viral infection will compromise the antiviral effect of IFN-α on infected cells (similar to the effect seen in Figs. 1 and 4) but that uninfected cells will remain sensitive to IFNs. These observations may also provide a key insight into the mechanism of antibody-dependent enhancement of viral infection, where it has been proposed that the antiviral effects of IFN-α/β are suppressed by Fc receptors and possibly by receptors for ssRNA (10). Further investigation of the role of PKC-dependent inhibition of IFNAR signaling in the development of IFN resistance and its relevance to the pathogenesis of viral infections is needed.
Acknowledgments
We thank Xianbo Zhou and Feng Cong for critical reading of the manuscript. This work was supported by National Institutes of Health Grants AI44938 (to L.B.I.), DK50693 (to B.G.N.), CA49152 (to B.G.N), and T32 AI07621 (to Z.D.).
Author contributions: L.B.I. designed research; Z.D. performed research; Y.S., W.Y., I.M., and B.G.N. contributed new reagents/analytic tools; Z.D. analyzed data; and Z.D. and L.B.I. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: IFNAR, type I IFN receptor; ssRNA, single-stranded RNA; TLR, Toll-like receptor; SOCS, suppressors of cytokine signaling; MAPK, mitogen-activated protein kinase; PMA, phorbol 12-myristate 13-acetate; FcγR, Fcγ receptor; RACK1, receptor for activated C-kinase 1.
References
- 1.Pestka, S., Langer, J. A., Zoon, K. C. & Samuel, C. E. (1987) Annu. Rev. Biochem. 56, 727-777. [DOI] [PubMed] [Google Scholar]
- 2.Bach, E. A., Kaplan, D. H. & Schrieber, R. D. (1999) in Inflammation: Basic Principles and Clinical Correlates, eds. Gallin, J. I. & Snyderman, R. (Lippincott Williams & Wilkins, Philadelphia), 3rd Ed., p. 487.
- 3.Stark, G. R., Kerr, I. M., Williams, B. R., Silverman, R. H. & Schreiber, R. D. (1998) Annu. Rev. Biochem. 67, 227-264. [DOI] [PubMed] [Google Scholar]
- 4.Darnell, J. E., Jr. (1997) Science 277, 1630-1635. [DOI] [PubMed] [Google Scholar]
- 5.Pearlman, B. L. (2004) Am. J. Med. 117, 344-352. [DOI] [PubMed] [Google Scholar]
- 6.Platanias, L. C. (1995) Curr. Opin. Oncol. 7, 560-565. [PubMed] [Google Scholar]
- 7.Brod, S. A. (2002) J. Interferon Cytokine Res. 22, 1153-1166. [DOI] [PubMed] [Google Scholar]
- 8.Pawlotsky, J. M. (2003) Curr. Opin. Infect. Dis. 16, 587-592. [DOI] [PubMed] [Google Scholar]
- 9.Gutterman, J. U. (1994) Proc. Natl. Acad. Sci. USA 91, 1198-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suhrbier, A. & La Linn, M. (2003) Trends Immunol. 24, 165-168. [DOI] [PubMed] [Google Scholar]
- 11.Ivashkiv, L. B. & Hu, X. (2003) Arthritis Rheum. 48, 2092-2096. [DOI] [PubMed] [Google Scholar]
- 12.Shuai, K. & Liu, B. (2003) Nat. Rev. Immunol. 3, 900-911. [DOI] [PubMed] [Google Scholar]
- 13.Yasukawa, H., Sasaki, A. & Yoshimura, A. (2000) Annu. Rev. Immunol. 18, 143-164. [DOI] [PubMed] [Google Scholar]
- 14.Ivashkiv, L. B. (2000) Rev. Immunogenet. 2, 220-230. [PubMed] [Google Scholar]
- 15.Ji, J. D., Tassiulas, I., Park-Min, K. H., Aydin, A., Mecklenbrauker, I., Tarakhovsky, A., Pricop, L., Salmon, J. E. & Ivashkiv, L. B. (2003) J. Exp. Med. 197, 1573-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed, S. T. & Ivashkiv, L. B. (2000) J. Immunol. 165, 5227-5237. [DOI] [PubMed] [Google Scholar]
- 17.Lee, I. H., Li, W. P., Hisert, K. B. & Ivashkiv, L. B. (1999) J. Exp. Med. 190, 1263-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sengupta, T. K., Talbot, E. S., Scherle, P. A. & Ivashkiv, L. B. (1998) Proc. Natl. Acad. Sci. USA 95, 11107-11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovanen, P. E., Junttila, I., Takaluoma, K., Saharinen, P., Valmu, L., Li, W. & Silvennoinen, O. (2000) Blood 95, 1626-1632. [PubMed] [Google Scholar]
- 20.Petricoin, E., III, David, M., Igarashi, K., Benjamin, C., Ling, L., Goelz, S., Finbloom, D. S. & Larner, A. C. (1996) Mol. Cell. Biol. 16, 1419-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen, V. A., Chen, J., Hong, F., Ishac, E. J. & Gao, B. (2000) Biochem. J. 349, 427-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu, J., Huang, H., Guo, L., Stonehouse, T., Watson, C. J., Hu-Li, J. & Paul, W. E. (2000) J. Exp. Med. 192, 1125-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bode, J. G., Schweigart, J., Kehrmann, J., Ehlting, C., Schaper, F., Heinrich, P. C. & Haussinger, D. (2003) J. Immunol. 171, 257-266. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed, S. T., Mayer, A., Ji, J. D. & Ivashkiv, L. B. (2002) J. Leukocyte Biol. 72, 154-162. [PubMed] [Google Scholar]
- 25.Zhang, S. Q., Tsiaras, W. G., Araki, T., Wen, G., Minichiello, L., Klein, R. & Neel, B. G. (2002) Mol. Cell. Biol. 22, 4062-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacGillivray, M., Herrera-Abreu, M. T., Chow, C. W., Shek, C., Wang, Q., Vachon, E., Feng, G. S., Siminovitch, K. A., McCulloch, C. A. & Downey, G. P. (2003) J. Biol. Chem. 278, 27190-27198. [DOI] [PubMed] [Google Scholar]
- 27.Rajagopal, R., Chen, Z. Y., Lee, F. S. & Chao, M. V. (2004) J. Neurosci. 24, 6650-6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alland, L., Peseckis, S. M., Atherton, R. E., Berthiaume, L. & Resh, M. D. (1994) J. Biol. Chem. 269, 16701-16705. [PubMed] [Google Scholar]
- 29.Heil, F., Hemmi, H., Hochrein, H., Ampenberger, F., Kirschning, C., Akira, S., Lipford, G., Wagner, H. & Bauer, S. (2004) Science 303, 1526-1529. [DOI] [PubMed] [Google Scholar]
- 30.Diebold, S. S., Kaisho, T., Hemmi, H., Akira, S. & Reis e Sousa, C. (2004) Science 303, 1529-1531. [DOI] [PubMed] [Google Scholar]
- 31.Melendez, A. J., Harnett, M. M. & Allen, J. M. (1999) Immunology 96, 457-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leitges, M., Schmedt, C., Guinamard, R., Davoust, J., Schaal, S., Stabel, S. & Tarakhovsky, A. (1996) Science 273, 788-791. [DOI] [PubMed] [Google Scholar]
- 33.Kermorgant, S., Zicha, D. & Parker, P. J. (2004) EMBO J. 23, 3721-3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hemmi, H., Kaisho, T., Takeuchi, O., Sato, S., Sanjo, H., Hoshino, K., Horiuchi, T., Tomizawa, H., Takeda, K. & Akira, S. (2002) Nat. Immunol. 3, 196-200. [DOI] [PubMed] [Google Scholar]
- 35.Guinamard, R., Signoret, N., Ishiai, M., Marsh, M., Kurosaki, T., Ravetch, J. V. & Masamichi, I. (1999) J. Exp. Med. 189, 1461-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castrillo, A., Pennington, D. J., Otto, F., Parker, P. J., Owen, M. J. & Bosca, L. (2001) J. Exp. Med. 194, 1231-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Croze, E., Usacheva, A., Asarnow, D., Minshall, R. D., Perez, H. D. & Colamonici, O. (2000) J. Immunol. 165, 5127-5132. [DOI] [PubMed] [Google Scholar]
- 38.David, M., Zhou, G., Pine, R., Dixon, J. E. & Larner, A. C. (1996) J. Biol. Chem. 271, 15862-15865. [DOI] [PubMed] [Google Scholar]
- 39.You, M., Yu, D. H. & Feng, G. S. (1999) Mol. Cell. Biol. 19, 2416-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strack, V., Krutzfeldt, J., Kellerer, M., Ullrich, A., Lammers, R. & Haring, H. U. (2002) Biochemistry 41, 603-608. [DOI] [PubMed] [Google Scholar]
- 41.Hong, F., Nguyen, V. A. & Gao, B. (2001) FASEB J. 15, 1595-1597. [DOI] [PubMed] [Google Scholar]
- 42.Uddin, S., Majchrzak, B., Woodson, J., Arunkumar, P., Alsayed, Y., Pine, R., Young, P. R., Fish, E. N. & Platanias, L. C. (1999) J. Biol. Chem. 274, 30127-30131. [DOI] [PubMed] [Google Scholar]
- 43.Sengupta, T. K., Schmitt, E. M. & Ivashkiv, L. B. (1996) Proc. Natl. Acad. Sci. USA 93, 9499-9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haro, T., Shimoda, K., Kakumitsu, H., Kamezaki, K., Numata, A., Ishikawa, F., Sekine, Y., Muromoto, R., Matsuda, T. & Harada, M. (2004) J. Immunol. 173, 1151-1157. [DOI] [PubMed] [Google Scholar]
- 45.Bacon, C. M., McVicar, D. W., Ortaldo, J. R., Rees, R. C., O'Shea, J. J. & Johnston, J. A. (1995) J. Exp. Med. 181, 399-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen, K. B., Watford, W. T., Salomon, R., Hofmann, S. R., Pien, G. C., Morinobu, A., Gadina, M., O'Shea, J. J. & Biron, C. A. (2002) Science 297, 2063-2066. [DOI] [PubMed] [Google Scholar]