Abstract
In mammals, intrinsically photosensitive retinal ganglion cells (ipRGCs) mediate non-image-forming visual functions such as pupillary light reflex (PLR) and circadian photoentrainment. This photosensitivity requires melanopsin, an invertebrate opsin-like protein expressed by the ipRGCs. The precise role of melanopsin remains uncertain. One suggestion has been that melanopsin may be a photoisomerase, serving to regenerate an unidentified pigment in ipRGCs. This possibility was echoed by a recent report that melanopsin is expressed also in the mouse retinal pigment epithelium (RPE), a key center for regeneration of rod and cone pigments. To address this question, we studied mice lacking RPE65, a protein essential for the regeneration of rod and cone pigments. Rpe65-/- ipRGCs were ≈20- to 40-fold less photosensitive than normal at both single-cell and behavioral (PLR) levels but were rescued by exogenous 9-cis-retinal (an 11-cis-retinal analog), indicating the requirement of a vitamin A-based chromophore for ipRGC photosensitivity. In contrast, 9-cis-retinal was unable to restore intrinsic photosensitivity to melanopsin-ablated ipRGCs, arguing against melanopsin functioning merely in photopigment regeneration. Interestingly, exogenous all-trans-retinal was also able to rescue the low sensitivity of rpe65-/- ipRGCs, suggesting that melanopsin could be a bistable pigment. Finally, we detected no melanopsin in the RPE and no changes in rod and cone sensitivities due to melanopsin ablation. Together, these results strongly suggest that melanopsin is the photopigment in the ipRGCs.
Keywords: RPE65, chromophore
Melanopsin, an opsin-like protein, was first identified in dermal melanophores of Xenopus laevis (1) and subsequently was found in a small subset of retinal ganglion cells that is intrinsically photosensitive in mammals (2, 3). These intrinsically photosensitive retinal ganglion cells (ipRGCs) project predominantly to the suprachiasmatic nucleus (SCN), the intergeniculate leaflet, and the olivary pretectal nucleus (OPN) of the brain (3). These nuclei are important for circadian photoentrainment and the pupillary light reflex (PLR), accessory visual functions reporting ambient luminance rather than image formation on the retina. In melanopsin-knockout (opn4-/-) mice, the ipRGCs are present in normal numbers and project to the correct targets in the brain but are no longer intrinsically photosensitive (4). Thus, melanopsin is required for light detection by these cells. The PLR of opn4-/- mice is also incomplete (4), and their circadian photoentrainment is attenuated (5, 6). In mice lacking both melanopsin and functional rods and cones, the PLR and circadian photoentrainment are abolished (7, 8). Thus, the melanopsin-associated and rod–cone photoreceptive systems appear to account for all major non-image-forming visual functions.
The action spectrum of the light response of ipRGCs can be fit by the absorption spectrum of a vitamin A-based photopigment with peak absorbance (λmax) at ≈484 nm (2). This action spectrum closely matches those for circadian photoentrainment and PLR by nonrod/noncone photoreceptors (8, 9). Nonetheless, the question of whether melanopsin is the signaling photopigment in ipRGCs remains. So far, the only functional study on heterologously expressed melanopsin has suggested an absorption spectrum with λmax at 424 nm (10), considerably shorter than that derived from native cells. This discrepancy and the fact that melanopsin shares only ≈27% amino acid identity with known vertebrate photopigments have led to the suggestion that melanopsin may not be the signaling pigment in question; instead, melanopsin may be a photoisomerase that photoconverts bound all-trans-retinal to 11-cis-retinal for regenerating an unidentified pigment in ipRGCs (11). 11-cis-retinal is the chromophore that binds covalently to rod and cone opsins to form functional photopigments. The possibility that melanopsin is a photoisomerase has gained ground recently when it was reported to be expressed also in the retinal pigment epithelium (RPE) (12), the primary site for 11-cis-retinal regeneration. Finally, adding to the confusion about melanopsin is a previous vitamin A-deprivation study reporting that ocular retinal is not required for light signaling to the SCN (13).
To investigate whether a vitamin A-based chromophore is required for photoreception by ipRGCs and to ask whether melanopsin is the signaling photopigment, we have studied a knockout mouse line that lacks RPE65, a key protein for regenerating 11-cis-retinal in the RPE (14–17). The level of 11-cis-retinal is practically undetectable in the retina of these animals. Their rod sensitivity is down by >103-fold (14, 18), and their cone electroretinogram (ERG) is undetectable (19). We examined the intrinsic photosensitivity of ipRGCs in this mouse line (rpe65-/-) before and after administration of exogenous 9-cis-retinal, an analog of 11-cis-retinal. To isolate the intrinsic light signals from ipRGCs in PLR measurements, we bred rpe65-/- mice into a gnat1-/-cnga3-/- background, which has nonfunctional rods and cones as a result of targeted deletions of the genes for the rod transducin α-subunit (gnat1) (20) and the cone cGMP-gated channel A subunit (cnga3) (21).
The results indicate that intrinsic photosignaling by the ipRGCs unequivocally requires a vitamin A-based chromophore. They also suggest that melanopsin is the signaling pigment.
Materials and Methods
Animals and X-Gal Labeling. Rpe65-/- mice, age-matched WT B6/129 F1 mice (Taconic), and albino CD-1 mice (Charles River Laboratories) were used. Rpe65-/-opn4+/-, rpe65-/-opn4-/-, rpe65-/-gnat1-/-cnga3-/-, and opn4-/- gnat1-/-cnga3-/- mice were generated by crossing existing lines (8). Typically, 1- to 3-month-old mice were used, but light-adapted ERGs were recorded from older animals.
X-Gal labeling of β-galactosidase activity in mice harboring opn4+/- or opn4-/- was performed as described in ref. 8 and is detailed in Supporting Text, which is published as supporting information on the PNAS web site.
Pupillometry. The procedure for measuring the consensual pupillary constriction was as described in ref. 8, with minor modifications detailed in Supporting Text.
i.p. Administration of 9-cis-Retinal and all-trans-Retinal. 9-cis-Retinal and all-trans-retinal were from Sigma-Aldrich. Animals were injected i.p. with 0.25 μg/g of body weight 9-cis-retinal or all-trans-retinal (0.05 μg/μl in 10% ethanol/10% BSA/0.9% NaCl) or vehicle under dim red light and were kept overnight in darkness before PLR measurement. Four- to 8-week-old rpe65-/-gnat1-/-cnga3-/- mice and 3- to 4-week-old rpe65-/- opn4-/- mice (younger to minimize rod degeneration) were used.
Whole-Cell Recording from IpRGCs. The experimental procedure, except for minor modifications, was as described in refs. 2 and 3. Details can be found in Supporting Text. The circular light spot (20-msec flashes at 480 nm, or white when much stronger light was necessary) on the retina was controlled by a field diaphragm and adjusted typically to 300 μm in diameter centered at the soma of the recorded cell. In all experiments, 0.1% ethanol was used throughout the recordings because one of the synaptic blockers, strychnine, used for isolating the intrinsic retinal ganglion cell light response was dissolved in ethanol. 9-cis-Retinal in a daily-made 10× stock solution containing 0.1% ethanol in Ames solution (22) was pipetted into the experimental chamber and left sitting for a few minutes before superfusion resumed and recording began. The final 9-cis-retinal concentration was ≈8 μg/ml, and the final total ethanol concentration was 0.11%. Ethanol concentration at 0.1% was reported to cause a transient small reduction in dark current and sensitivity for rod and cones (22).
Results
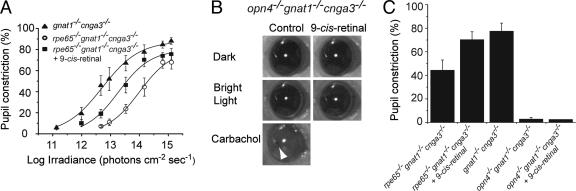
PLR of Rpe65-/-Gnat1-/-Cnga3-/- Mice. We studied the effect of ablating RPE65 on the PLR to check for any dependence of ipRGC function on this protein. To remove rod–cone signals in the animal, we bred rpe65-/- mice into a gnat1-/-cnga3-/- line, which has nonfunctional rods and cones (see the Introduction). At 1–2 months of age, the triple-knockout mouse had normal-looking retinal morphology, similar to that of the gnat1-/- cnga3-/- mouse (Fig. 1A). This intactness of the retina is consistent with a recent study that showed that the retinal degeneration caused by ablating RPE65 can be rescued by genetically preventing rods from signaling (23). When probed with 480-nm light, the PLR of rpe65-/-gnat1-/-cnga3-/- mice showed an irradiance–response relation shifted to higher intensities by 1.4 log units (25-fold) than that of gnat1-/-cnga3-/- mice (Fig. 2A), indicating a substantially reduced ipRGC sensitivity without RPE65. The maximal constriction also appeared smaller. These results immediately suggest that ipRGC signaling depends on 11-cis-retinal.
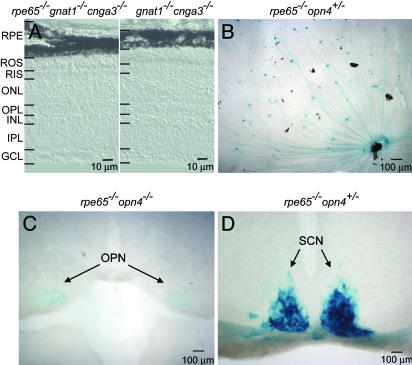
Fig. 1.
Retinal morphology and axonal projections of ipRGCs in the absence of RPE65. (A) Retinal cross sections from gnat1-/-cnga3-/- and rpe65-/- gnat1-/-cnga3-/- mice. ROS, rod outer segment; RIS, rod inner segment; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. (B) Flat-mounted retina from an rpe65-/-opn4+/- mouse stained with X-Gal (blue). (C and D) Coronal sections of rpe65-/-opn4-/- (C) and rpe65-/-opn4+/- (D) mouse brains showing the X-Gal-labeled ipRGC axons projecting to the OPN and SCN. Dorsal side is up in each case. The rpe65-/-opn4-/- mouse instead of the rpe65-/-opn4+/- mouse was used in C to give a more intense X-Gal labeling of the OPN.
Fig. 2.
PLR of mice with different genotypes. (A) Irradiance–response relations for the PLR of gnat1-/-cnga3-/-, rpe65-/-gnat1-/-cnga3-/-, and 9-cis-retinal-treated rpe65-/-gnat1-/-cnga3-/- mice with steady light (480 nm). Fractional pupil constriction was calculated as 1 - (pupil area in light/dark-adapted pupil area). The best-fit curves fitted to the data were calculated from 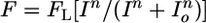 , where FL is the maximal percentage pupil constriction in bright light (a parameter in the fit), I is irradiance, Io is a constant, and n is the Hill coefficient. Note that FL is generally not 100% because the pupil area does not go down to zero even in very bright light. The parameters for the fits are as follows: FL = 88.0%, Io = 1012.71, n = 0.68 (gnat1-/-cnga3-/-); FL = 79.5%, Io = 1014.0, n = 0.78 (rpe65-/-gnat1-/-cnga3-/-); FL = 79.7%, Io = 1013.3, n = 0.78 (rpe65-/-gnat1-/-cnga3-/- plus 9-cis-Retinal). (B) 9-cis-Retinal had no effect on the PLR of opn4-/-gnat1-/-cnga3-/- mice, whereas topical application of 100 mM carbachol was able to produce a complete PLR (arrowhead indicates pupil). (C) Collected results on PLR for different genetic lines in bright light. Note that 9-cis-retinal was unable to rescue the PLR of opn4-/-gnat1-/-cnga3-/- mice (n = 6). Data on rpe65-/-gnat1-/-cnga3-/- and gnat1-/-cnga3-/- mice were from A. Irradiance was 480 nm and 1.6 × 1014 photons·cm-2·sec-1. For opn4-/-gnat1-/-cnga3-/- mice plus 9-cis-retinal, a light 10-fold brighter still produced no improvement in PLR over untreated opn4-/-gnat1-/-cnga3-/- mice (data not shown). Data are mean ± SEM (n = 4–7).
, where FL is the maximal percentage pupil constriction in bright light (a parameter in the fit), I is irradiance, Io is a constant, and n is the Hill coefficient. Note that FL is generally not 100% because the pupil area does not go down to zero even in very bright light. The parameters for the fits are as follows: FL = 88.0%, Io = 1012.71, n = 0.68 (gnat1-/-cnga3-/-); FL = 79.5%, Io = 1014.0, n = 0.78 (rpe65-/-gnat1-/-cnga3-/-); FL = 79.7%, Io = 1013.3, n = 0.78 (rpe65-/-gnat1-/-cnga3-/- plus 9-cis-Retinal). (B) 9-cis-Retinal had no effect on the PLR of opn4-/-gnat1-/-cnga3-/- mice, whereas topical application of 100 mM carbachol was able to produce a complete PLR (arrowhead indicates pupil). (C) Collected results on PLR for different genetic lines in bright light. Note that 9-cis-retinal was unable to rescue the PLR of opn4-/-gnat1-/-cnga3-/- mice (n = 6). Data on rpe65-/-gnat1-/-cnga3-/- and gnat1-/-cnga3-/- mice were from A. Irradiance was 480 nm and 1.6 × 1014 photons·cm-2·sec-1. For opn4-/-gnat1-/-cnga3-/- mice plus 9-cis-retinal, a light 10-fold brighter still produced no improvement in PLR over untreated opn4-/-gnat1-/-cnga3-/- mice (data not shown). Data are mean ± SEM (n = 4–7).
To eliminate the possibility that the above decreased PLR sensitivity was caused by fewer ipRGCs or misprojection of the ipRGCs to the brain, we produced rpe65-/-opn4+/- and rpe65-/-opn4-/- mice to visualize the cells and their projections by labeling with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactoside) for the β-galactosidase coded by the tau-lacZ marker gene in opn4+/- and opn4-/- mice (3, 4). The ipRGCs were present in normal number (≈600 per retina) (Fig. 1B) and projected to the OPN (the control center for the PLR) and SCN normally (Fig. 1 C and D). Thus, the genesis and projections of the ipRGCs are unaffected by ablating RPE65.
Effect of Exogenous 9-cis-Retinal on the PLR of Rpe65-/-Gnat1-/-Cnga3-/- Mice. Others have found that the low rod sensitivity of rpe65-/- mice can be largely rescued by exogenous retinal (18, 24, 25). We checked for the same in ipRGCs with 9-cis-retinal, a widely used isomer of 11-cis-retinal that generates functional visual pigments (26–28). Indeed, at 16 hr after an i.p. injection of 9-cis-retinal (0.25 μg/g of body weight; see Materials and Methods) into rpe65-/-gnat1-/-cnga3-/- mice, the irradiance–response relation of the PLR shifted to lower light intensities by ≈0.7 log units (5-fold), midway between those for gnat1-/- cnga3-/- mice and uninjected rpe65-/-gnat1-/-cnga3-/- mice (Fig. 2 A). Thus, the signaling pigment in the ipRGCs is able to use exogenous retinal as chromophore, confirming that it is a vitamin A-based pigment.
If melanopsin is not the signaling pigment but is merely a photoisomerase critically necessary for chromophore regeneration in ipRGCs, one should expect exogenous retinal to likewise rescue the PLR of opn4-/-gnat1-/-cnga3-/- mice. However, i.p. injection of 9-cis-retinal into these mice failed to have any effect (Fig. 2 B and C). This lack of effect was not due to an intrinsic defect in the iris sphincter of opn4-/-gnat1-/-cnga3-/- animals, because parasympathetic activation by topical application of carbachol was able to elicit a full constriction (Fig. 2B). Thus, the rescue of the PLR of rpe65-/-gnat1-/-cnga3-/- mice by 9-cis-retinal requires melanopsin.
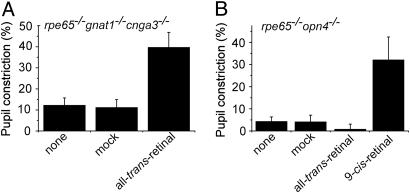
Effect of Exogenous all-trans-Retinal on the PLR of Rpe65-/-Gnat1-/-Cnga3-/- Mice. Melanopsin shares significant sequence homology to invertebrate opsins (1), suggesting that it may function as a bistable pigment (i.e., with the dual function of a photopigment and a photoisomerase). To test this possibility, we injected all-trans-retinal i.p. into rpe65-/-gnat1-/-cnga3-/- mice. These animals are unable to convert all-trans-retinal to 11-cis-retinal because of loss of RPE65 (14). Such animals treated with all-trans-retinal and maintained in darkness nonetheless showed a >3-fold increase in PLR sensitivity (Fig. 3A). In sharp contrast, all-trans-retinal failed to improve the PLR in 1-month-old or younger rpe65-/-opn4-/- mice (Fig. 3B), which have no melanopsin but still retain rods. This control experiment confirmed that all-trans-retinal (unlike 9-cis-retinal; see Fig. 3B) was unable to restore rod sensitivity. Thus, unlike rods, ipRGCs are able to use all-trans-retinal for detecting light.
Fig. 3.
Effect of all-trans-retinal on the PLR. i.p. injection of all-trans-retinal rescued the PLR of rpe65-/-gnat1-/-cnga3-/- mice (A) but not of rpe65-/- opn4-/- mice (B). As a positive control, 9-cis-retinal improved the PLR of rpe65-/-opn4-/- mice. Irradiance was 480 nm and 2.4 × 1013 and 3.2 × 1012 photons·cm-2·sec-1 in A and B, respectively. Data are mean ± SEM (n = 12 in A, and n = 4 in B).
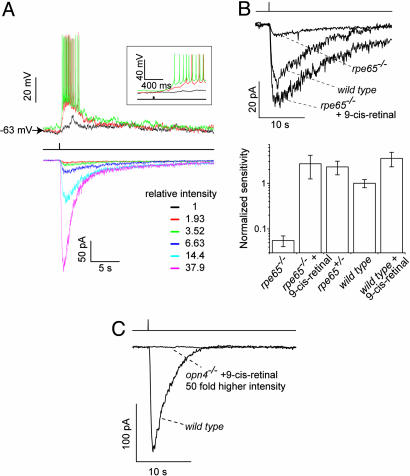
Single-Cell Recordings from IpRGCs in Rpe65-/- Mice. We measured directly the intrinsic light response of single ipRGCs in the isolated retina by removing rod–cone signals with synaptic blockers (see Materials and Methods) (2, 3). These blockers eliminated all spontaneous postsynaptic currents recorded from these cells in darkness (data not shown), suggesting an effective removal of rod–cone signals. Fig. 4A shows typical intrinsic photoresponses from an rpe65+/- ipRGC under current clamp and voltage clamp, respectively. The flash sensitivity of rpe65-/- ipRGCs was, on average, 20 to 40 times lower than WT or rpe65+/- ipRGCs (Fig. 4B). This sensitivity change is similar to that for the PLR due to rpe65-/- in a gnat1-/-cnga3-/- background (compare Fig. 2 A). After superfusion of the rpe65-/- retina with 9-cis-retinal (≈8 μg/ml) for a few minutes, however, the normal flash sensitivity of rpe65-/- ipRGCs was restored (Fig. 4B). The small increase in sensitivity of WT ipRGCs also caused by 9-cis-retinal (Fig. 4B) was probably due to some opsin molecules somehow rendered devoid of chromophore under our experimental conditions. The complete restoration in sensitivity of in vitro rpe65-/- ipRGCs, versus partial restoration of the PLR in rpe65-/-gnat1-/-cnga3-/- mice, likely reflected a better delivery of 9-cis-retinal to the ipRGCs by direct superfusion of the retina than by i.p. injection into the animal.
Fig. 4.
Light responses and sensitivities of single rpe65+/-, rpe65-/-, and opn4-/- ipRGCs. (A) Flash responses recorded from a representative ipRGC in an rpe65+/- retina under current-clamp (Upper) and voltage-clamp (Lower, -70 mV) modes, respectively. Single flash trials are shown. The same stimulus was used in both cases: a 20-msec, 480-nm flash (middle trace) of the indicated relative intensities focused on a 300-μm spot centered at the soma. Not all indicated intensities were used in the current-clamp and voltage-clamp recordings. (Inset) The same current-clamp records on a faster time base. The light spot did not cover all distal dendrites of the cell. (B) Rescue of photosensitivity of rpe65-/- ipRGC by exogenous 9-cis-retinal. (Upper) Representative flash responses of WT, rpe65-/-, and 9-cis-retinal-treated rpe65-/- ipRGCs. The same flash intensity and single trials were used in all three cases. (Lower) Collected results on relative flash sensitivity from various mouse lines and manipulations. Values relative to WT are as follows (mean ± SEM): 0.056 ± 0.015 (rpe65-/-, n = 12); 2.7 ± 1.4 (rep65-/- plus 9-cis-retinal, n = 5); 2.3 ± 0.8 (rpe65+/-, n = 7); 1.0 ± 0.2 (WT, n = 12); 3.5 ± 1.2 (WT plus 9-cis-retinal, n = 6). See Materials and Methods. (C) 9-cis-Retinal was unable to restore the sensitivity of opn4-/- ipRGCs. Representative recordings from opn4-/- and WT ipRGCs are shown. Light responses by opn4-/- ipRGCs (n = 7) were never observed with or without 9-Cis-retinal, even for the strongest light.
In contrast, ipRGCs in opn4-/- retina failed to show any light response after superfusion with 9-cis-retinal (Fig. 4C). This result corroborated the above finding that 9-cis-retinal was unable to restore any PLR to opn4-/-gnat1-/-cnga3-/- mice, strongly suggesting that melanopsin is the signaling pigment in ipRGCs (see Discussion).
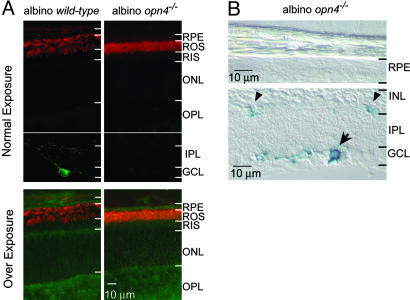
Rod and Cone Sensitivities of Opn4-/- and WT Mice. Melanopsin has recently been reported to be expressed in the mouse RPE, strengthening a persistently raised possibility that it functions in chromophore regeneration (12). We first sought to confirm by immunofluorescence the expression of melanopsin in the RPE, but we failed to detect any convincing signal from WT (B6/129) mice (data not shown). To rule out any masking of melanopsin immunofluorescence in the RPE by the intrinsic pigmentation of these cells, we repeated the immunolabeling with an albino mouse line (CD-1). Nonetheless, we still did not detect any melanopsin immunofluorescence (green color) in the RPE (Fig. 5A Upper); the apparent labeling on prolonged exposure was probably nonspecific because it was also present in the opn4-/- retina with the same albino background (green color in Fig. 5A Lower). As a complementary approach, we checked for X-Gal labeling of the RPE in the albino opn4-/- mouse, which contains the tau-LacZ marker gene, but we did not detect any labeling (Fig. 5B). We conclude that the RPE expresses either no melanopsin or an undetectable amount of melanopsin.
Fig. 5.
Immunocytochemistry and X-Gal labeling of retinal cross sections from albino WT and albino opn4-/- mice. (A) Double labeling with an antibody against the N terminus of melanopsin (3) (green) and the anti-rhodopsin 1D4 monoclonal antibody (red). The rhodopsin immunofluorescence helped in identifying the adjacent RPE layer by showing the rod outer segments. Upon normal frame exposure, melanopsin-expressing ipRGC is visible in WT inner retina, but no melanopsin signal is detectable in the RPE layer. Upon overexposure, punctate green signal is present in the RPE layer (and elsewhere) of WT and opn4-/- mice, suggesting that the signal is nonspecific. (B) X-Gal labeling of albino opn4-/- retinal cross sections. The blue labeling is absent in the RPE but present in the ipRGC (arrow), and its processes are present in the inner plexiform layer (arrowheads). The abbreviations for retinal layers are as in Fig. 1.
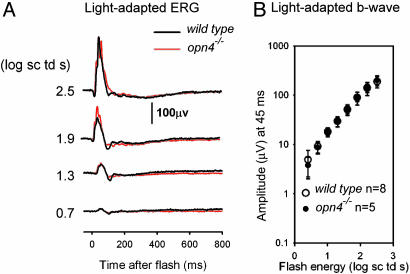
To check whether any undetectable amount of melanopsin in the RPE may still contribute to rod and cone sensitivities, we examined their sensitivities with opn4-/- mice. In suction-pipette recordings, the flash sensitivity and response kinetics of single rods isolated from overnight-dark-adapted opn4-/- and WT mice were very similar, arguing against melanopsin loss having an impact on rod pigment regeneration in darkness (see Table 1, which is published as supporting information on the PNAS web site). We also measured the ERG. Likewise, we observed no difference between WT and opn4-/- mice in the photopic ERG (Fig. 6A) or the intensity–response relation of the photopic b-wave (Fig. 6B). Scotopic ERGs elicited by 462-nm flashes in the two mouse lines were also very similar (see Fig. 7 A and B, which is published as supporting information on the PNAS web site), consistent with the suction-pipette experiments. Finally, we examined the effect of background light ranging from weak to saturating for rods and found no difference between the two types of animals (data not shown). Thus, there is no evidence that melanopsin is present in the RPE or affects the sensitivity of rods or cones.
Fig. 6.
ERG responses of opn4-/- and WT mice. (A) Light-adapted ERGs measured in a steady background of 2.5 log scotopic troland, sufficient to saturate the rod response. (B) Intensity–response relations for the light-adapted b-wave. The b-wave amplitude was measured at 45 msec after the flash, when the positive-going wave was maximal over most of the stimulus range (see A). The eight WT mice indicated included seven C57BL6 animals, owing to the reduced sample of WT B6/129 mice.
Discussion
The restoration of the PLR of rpe65-/- animals (in a genetic background of nonfunctional rods and cones) by i.p. injection of 9-cis-retinal, together with the restoration of rpe65-/- ipRGC photosensitivity by exogenous 9-cis-retinal, provides direct evidence that the signaling pigment in ipRGCs uses a vitamin A-based chromophore. This conclusion contradicts a previous claim by others that ocular retinal is not required for signaling of light to the SCN (13). This earlier study found that mice with barely detectable levels of ocular retinal (through vitamin A deprivation) retained normal Per induction in the SCN in response to brief light pulses. Presumably, the low but finite amount of ocular retinal in these animals was below detection (see also next paragraph). Also, Per induction may not track sensitivity changes in the ipRGCs as quantitatively as the PLR or direct electrical recordings used in the present study. Interestingly, a recent report (29) also described some persistent, albeit much less sensitive, PLR in rpe65-/- mice but did not adequately comment on the vitamin A-deprivation work. One uncertainty in this report (29) is that the impact of RPE65 ablation on the ipRGCs could not be well defined, because it leads to severe impairment of rod and cone functions and to retinal degeneration. Our study, in contrast, uses the gnat1-/- cnga3-/- genetic background, which avoids degeneration of the retina while also allowing specific study of the rpe65-/- effect on the ipRGCs.
Owing to a disruption in chromophore regeneration, the eyes of the rpe65-/- mouse have no detectable levels of 11-cis-retinal except for an elevated level of all-trans-retinyl esters, as assayed by HPLC analysis (14). The level of holopigment in rpe65-/- rods was estimated to be <0.1% of WT (C57BL/6) (0.2 pmol per retina) (14, 18, 24, 25), based on the detection limit of HPLC and the failure to observe any light absorption even after pooling retinal tissue from multiple animals (25, 30). Correspondingly, rod sensitivity decreases by >103-fold (14, 18). In comparison, we found that the photosensitivity of rpe65-/- ipRGCs decreased by only 20 to 40 times. Without any direct measurement of holopigment content in rpe65-/- ipRGCs or any knowledge of their phototransduction mechanism, it is difficult to determine the percentage decrease in ipRGC holopigment due to the absence of RPE65. However, because the decrease in sensitivity is much smaller in ipRGC than in rods, it is quite possible that the decrease in holopigment in ipRGCs is considerably less than that experienced by the rod pigment. Thus, although the signaling pigment in ipRGCs uses a vitamin A-based chromophore, this pigment appears less susceptible to defective 11-cis-retinal regeneration in the RPE (see below), which may explain why rpe65-/- mice can still be fully photoentrained (31). Finally, because the population of ipRGCs is small (≈600 cells per mouse retina), its contribution to the overall retinoid content in the eye is negligible, thus explaining its lack of detectability in the vitamin A-deprivation study quoted above.
Our finding that 9-cis-retinal is incapable of restoring any photosensitivity to the opn4-/- ipRGCs or any PLR to the opn4-/- gnat1-/-cnga3-/- mouse rules out the possibility that another opsin exists in these cells and functions as the signaling pigment. The ipRGC in an opn4-/- background could not simply have lost its photosignaling ability due to chromophore loss during development, because the ipRGCs in rpe65-/- mice, also with chromophore loss, can be rescued. Thus, unless melanopsin has a highly unconventional function for an opsin-like protein (namely, merely delivering 11-cis-retinal to a signaling pigment) it appears that melanopsin is the bona fide signaling pigment itself.
all-trans-Retinal is able to restore the photosensitivity of rpe65-/-gnat1-/-cnga3-/- mice but not rpe65-/-opn4-/- mice, suggesting that ipRGCs can regenerate their pigment autonomously through conversion of all-trans-retinal to 11-cis-retinal. One possibility is that ipRGCs have an endogenous photoisomerase for the chromophore in addition to expressing melanopsin as the signaling pigment. A more parsimonious scenario is that melanopsin serves the dual function of the signaling pigment and the photoisomerase (i.e., it is a bistable pigment). The conversion of all-trans-retinal to 11-cis-retinal in our experiments could happen during the 1-min light stimulus or even through slow dark regeneration as documented for some invertebrate bistable pigments (32). The putative bistability can explain why the ipRGC photopigment appears less susceptible to the absence of RPE65 than rod and cone pigments. In this regard, it is tantalizing that, phylogenetically, melanopsin shows more kinship to invertebrate pigments (1), some of which are bistable, than to rod and cone pigments. Conceivably, a bistable melanopsin in the ipRGCs would be able to function continuously despite being physically far removed from the RPE. The reduced ipRGC sensitivity in rpe65-/- mice suggests that melanopsin still depends on RPE for its initial supply of chromophore. Indeed, in the rpe65-/- background, melanopsin has to compete with the overwhelmingly abundant rod opsin (which acts as a huge sink) for the very limited supply of chromophore. This competition may explain why melanopsin, even if bistable, cannot retain sufficient chromophore in rpe65-/- animals for normal function. The pathway by which 11-cis-retinal finds its way from the RPE to the ipRGCs remains unclear. One possible conduit is the Müller cells, which span the full thickness of the retina.
Mutations in RPE65 account for ≈10% of childhood-onset retinal degenerations known as Leber's congenital amaurosis (LCA) and some cases of recessive retinitis pigmentosa (33–35). Rpe65-/- mice have proven to be a valuable animal model for studying the mechanism and the treatment of this disease (14, 18, 24, 25). The data presented here suggest that the loss of RPE65, or its defects, may also be detrimental (at least partially) to the non-image-forming visual functions in these LCA patients. The rod–cone vision of rpe65-/- mice can be rescued by exogenous 9-cis-retinal or 11-cis-retinal, suggesting a potential therapy for restoring vision in these LCA patients (18, 24, 25, 36). The same may apply to the non-image-forming visual functions.
Note. After the completion of this work, several studies based on heterologous-expression experiments appeared, reporting that melanopsin is indeed a signaling pigment, although there was no unanimity about whether melanopsin is bistable (37–39). Our current work provides a complementary approach by focusing on the native retina and ipRGCs.
Supplementary Material
Acknowledgments
We thank Y. Liang for mouse genotyping, Dr. J. Ma (University of Oklahoma, Oklahoma City) for the rpe65-/- mice originally generated by Dr. T Michael Redmond (National Institutes of Health, Bethesda), Dr. J. Lem (Tufts University, Boston) for gnat1-/- mice, Dr. M. Biel (Universität München, Munich) for cnga3-/- mice, Dr. R. S. Molday (University of British Columbia, Vancouver) for the 1D4 antibody, and T. Shelley for constructing the pupillometer. We also thank members of K.-W.Y.'s laboratory for discussions and comments on the manuscript. This work was supported by National Eye Institute Grants EY06837 and EY14596 (to K.-W.Y.) and EY06671 (to L.J.F.).
Author contributions: Y.F., H.Z., M.-H.H.W., D.-G.L., H.M., L.J.F., and K.-W.Y. designed research; Y.F., H.Z., M.-H.H.W., D.-G.L., H.-W.L., and H.M. performed research; S.H. contributed new reagents/analytic tools; Y.F., H.Z., M.-H.H.W., D.-G.L., H.M., and L.J.F. analyzed data; and Y.F., H.Z., L.J.F., and K.-W.Y. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ipRGC, intrinsically photosensitive retinal ganglion cell; PLR, pupillary light reflex; ERG, electroretinogram; RPE, retinal pigment epithelium; SCN, suprachiasmatic nucleus; OPN, olivary pretectal nucleus.
References
- 1.Provencio, I., Jiang, G., De Grip, W. J., Hayes, W. P. & Rollag, M. D. (1998) Proc. Natl. Acad. Sci. USA 95, 340-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berson, D. M., Dunn, F. A. & Takao, M. (2002) Science 295, 1070-1073. [DOI] [PubMed] [Google Scholar]
- 3.Hattar, S., Liao, H. W., Takao, M., Berson, D. M. & Yau, K. W. (2002) Science 295, 1065-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucas, R. J., Hattar, S., Takao, M., Berson, D. M., Foster, R. G. & Yau, K. W. (2003) Science 299, 245-247. [DOI] [PubMed] [Google Scholar]
- 5.Ruby, N. F., Brennan, T. J., Xie, X., Cao, V., Franken, P., Heller, H. C. & O'Hara, B. F. (2002) Science 298, 2211-2213. [DOI] [PubMed] [Google Scholar]
- 6.Panda, S., Sato, T. K., Castrucci, A. M., Rollag, M. D., DeGrip, W. J., Hogenesch, J. B., Provencio, I. & Kay, S. A. (2002) Science 298, 2213-2216. [DOI] [PubMed] [Google Scholar]
- 7.Panda, S., Provencio, I., Tu, D. C., Pires, S. S., Rollag, M. D., Castrucci, A. M., Pletcher, M. T., Sato, T. K., Wiltshire, T., Andahazy, M., et al. (2003) Science 301, 525-527. [DOI] [PubMed] [Google Scholar]
- 8.Hattar, S., Lucas, R. J., Mrosovsky, N., Thompson, S., Douglas, R. H., Hankins, M. W., Lem, J., Biel, M., Hofmann, F., Foster, R. G. & Yau, K. W. (2003) Nature 424, 76-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucas, R. J., Douglas, R. H. & Foster, R. G. (2001) Nat. Neurosci. 4, 621-626. [DOI] [PubMed] [Google Scholar]
- 10.Newman, L. A., Walker, M. T., Brown, R. L., Cronin, T. W. & Robinson, P. R. (2003) Biochemistry 42, 12734-12738. [DOI] [PubMed] [Google Scholar]
- 11.Foster, R. G. & Bellingham, J. (2004) Photochem. Photobiol. Sci. 3, 617-627. [DOI] [PubMed] [Google Scholar]
- 12.Peirson, S. N., Bovee-Geurts, P. H., Lupi, D., Jeffery, G., DeGrip, W. J. & Foster, R. G. (2004) Brain Res. Mol. Brain Res. 123, 132-135. [DOI] [PubMed] [Google Scholar]
- 13.Thompson, C. L., Blaner, W. S., Van Gelder, R. N., Lai, K., Quadro, L., Colantuoni, V., Gottesman, M. E. & Sancar, A. (2001) Proc. Natl. Acad. Sci. USA 98, 11708-11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redmond, T. M., Yu, S., Lee, E., Bok, D., Hamasaki, D., Chen, N., Goletz, P., Ma, J. X., Crouch, R. K. & Pfeifer, K. (1998) Nat. Genet. 20, 344-351. [DOI] [PubMed] [Google Scholar]
- 15.Mata, N. L., Moghrabi, W. N., Lee, J. S., Bui, T. V., Radu, R. A., Horwitz, J. & Travis, G. H. (2004) J. Biol. Chem. 279, 635-643. [DOI] [PubMed] [Google Scholar]
- 16.Gollapalli, D. R., Maiti, P. & Rando, R. R. (2003) Biochemistry 42, 11824-11830. [DOI] [PubMed] [Google Scholar]
- 17.Xue, L., Gollapalli, D. R., Maiti, P., Jahng, W. J. & Rando, R. R. (2004) Cell 117, 761-771. [DOI] [PubMed] [Google Scholar]
- 18.Van Hooser, J. P., Aleman, T. S., He, Y. G., Cideciyan, A. V., Kuksa, V., Pittler, S. J., Stone, E. M., Jacobson, S. G. & Palczewski, K. (2000) Proc. Natl. Acad. Sci. USA 97, 8623-8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seeliger, M. W., Grimm, C., Stahlberg, F., Friedburg, C., Jaissle, G., Zrenner, E., Guo, H., Reme, C. E., Humphries, P., Hofmann, F., et al. (2001) Nat. Genet. 29, 70-74. [DOI] [PubMed] [Google Scholar]
- 20.Calvert, P. D., Krasnoperova, N. V., Lyubarsky, A. L., Isayama, T., Nicolo, M., Kosaras, B., Wong, G., Gannon, K. S., Margolskee, R. F., Sidman, R., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 13913-13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biel, M., Seeliger, M., Pfeifer, A., Kohler, K., Gerstner, A., Ludwig, A., Jaissle, G., Fauser, S., Zrenner, E. & Hofmann, F. (1999) Proc. Natl. Acad. Sci. USA 96, 7553-7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cornwall, M. C., Jones, G. J., Kefalov, V. J., Fain, G. L. & Matthews, H. R. (2000) Methods Enzymol. 316, 224-252. [DOI] [PubMed] [Google Scholar]
- 23.Woodruff, M. L., Wang, Z., Chung, H. Y., Redmond, T. M., Fain, G. L. & Lem, J. (2003) Nat. Genet. 35, 158-164. [DOI] [PubMed] [Google Scholar]
- 24.Van Hooser, J. P., Liang, Y., Maeda, T., Kuksa, V., Jang, G. F., He, Y. G., Rieke, F., Fong, H. K., Detwiler, P. B. & Palczewski, K. (2002) J. Biol. Chem. 277, 19173-19182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ablonczy, Z., Crouch, R. K., Goletz, P. W., Redmond, T. M., Knapp, D. R., Ma, J. X. & Rohrer, B. (2002) J. Biol. Chem. 277, 40491-40498. [DOI] [PubMed] [Google Scholar]
- 26.Hubbard, R. & Wald, G. (1952) J. Gen. Physiol. 36, 269-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pepperberg, D. R., Brown, P. K., Lurie, M. & Dowling, J. E. (1978) J. Gen. Physiol. 71, 369-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corson, D. W., Cornwall, M. C., MacNichol, E. F., Mani, V. & Crouch, R. K. (1990) Biophys. J. 57, 109-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batten, M. L., Imanishi, Y., Maeda, T., Tu, D. C., Moise, A. R., Bronson, D., Possin, D., Van Gelder, R. N., Baehr, W. & Palczewski, K. (2004) J. Biol. Chem. 279, 10422-10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan, J., Rohrer, B., Moiseyev, G., Ma, J. X. & Crouch, R. K. (2003) Proc. Natl. Acad. Sci. USA 100, 13662-13667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daniels, D. M., Stoddart, C. W., Martin-Iverson, M. T., Lai, C. M., Redmond, T. M. & Rakoczy, P. E. (2003) Physiol. Behav. 79, 701-711. [DOI] [PubMed] [Google Scholar]
- 32.Hillman, P., Hochstein, S. & Minke, B. (1983) Physiol. Rev. 63, 668-772. [DOI] [PubMed] [Google Scholar]
- 33.Gu, S. M., Thompson, D. A., Srikumari, C. R., Lorenz, B., Finckh, U., Nicoletti, A., Murthy, K. R., Rathmann, M., Kumaramanickavel, G., Denton, M. J. & Gal, A. (1997) Nat. Genet. 17, 194-197. [DOI] [PubMed] [Google Scholar]
- 34.Marlhens, F., Bareil, C., Griffoin, J. M., Zrenner, E., Amalric, P., Eliaou, C., Liu, S. Y., Harris, E., Redmond, T. M., Arnaud, B., et al. (1997) Nat. Genet. 17, 139-141. [DOI] [PubMed] [Google Scholar]
- 35.Morimura, H., Fishman, G. A., Grover, S. A., Fulton, A. B., Berson, E. L. & Dryja, T. P. (1998) Proc. Natl. Acad. Sci. USA 95, 3088-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aleman, T. S., Jacobson, S. G., Chico, J. D., Scott, M. L., Cheung, A. Y., Windsor, E. A., Furushima, M., Redmond, T. M., Bennett, J., Palczewski, K. & Cideciyan, A. V. (2004) Invest. Ophthalmol. Visual Sci. 45, 1259-1271. [DOI] [PubMed] [Google Scholar]
- 37.Qiu, X., Kumbalasiri, T., Carlson, S. M., Wong, K. Y., Krishna, V., Provencio, I. & Berson, D. M. (2005) Nature 433, 745-749. [DOI] [PubMed] [Google Scholar]
- 38.Panda, S., Nayak, S. K., Campo, B., Walker, J. R., Hogenesch, J. B. & Jegla, T. (2005) Science 307, 600-604. [DOI] [PubMed] [Google Scholar]
- 39.Melyan, Z., Tarttelin, E. E., Bellingham, J., Lucas, R. J. & Hankins, M. W. (2005) Nature 433, 741-745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.