Abstract
Multiple sclerosis (MS) is a complex disease that seems to depend on several pathophysiological processes. Because of its varied clinical presentation, natural history, and response to therapeutic interventions, MS can be considered to be a group of diseases that have not been yet characterized, thus resulting in difficult evaluation of prognosis. In the last few years, the role of autoAbs in MS has been reevaluated, and, therefore, their identification as specific biomarkers became a relevant target. In this paper, we demonstrate that an aberrant N-glucosylation is a fundamental determinant of autoAb recognition in MS. Thus, we developed CSF114(Glc), an antigenic probe accurately measuring IgM autoAbs in the sera of a patient population, as disease biomarker. The relevance of CSF114(Glc) is demonstrated by its clinical application and correlation with disease activity and prognosis. In fact, CSF114(Glc), a structure-based designed glycopeptide, is able to recognize, by ELISA, the presence of specific IgM autoAbs in the sera of a MS patient population but not in blood donors and other autoimmune conditions. AutoAbs specific for CSF114(Glc) isolated from MS patients recognized myelin and oligodendrocyte antigens by immunohistochemistry but not other nonrelevant tissues. We demonstrate that CSF114(Glc) is a reliable, specific probe in a longitudinal study of untreated MS patients. Development of IgG/IgM anti-CSF114(Glc) Abs paralleled clinical activity and brain lesions positive to MRI. Therefore, a CSF114(Glc)-based immunoassay on sera may have important prognostic value in monitoring MS disease progression guiding optimal therapeutic treatment.
Keywords: aberrant glycosylation, prognostic probe, synthetic antigen, β-hairpin
Multiple sclerosis (MS), the most frequent chronic inflammatory demyelinating disease of the CNS, is the most common cause of disability in young adults. Most cases are diagnosed between the ages of 20 and 40, with a higher incidence in women. It is estimated that it affects over one million people worldwide. Cruelly, although not considered a life-shortening disease, MS tends to strike people at the prime of their lives, often interrupting or even terminating their careers (1). Therefore, the disease has a large social impact, with spiraling costs increasing with the progression of disability (2). If progression can be delayed, the quality of life and independence of MS patients will improve and the cost to health care system and society will decrease accordingly (3). Thus, an early prognosis could facilitate such a delay in progression.
Although an autoimmune mechanism against CNS myelin antigens (Ags) is thought to contribute to the immunopathological mechanism of the disease, the target Ags remain elusive. MS is an heterogeneous disease, particularly in its clinical subforms, with an extremely variable evolution over time. Because of its varied clinical presentation, natural history, and response to therapeutic interventions, MS may be considered to be a group of diseases. Lucchinetti et al. (4) proposed four distinct patterns of MS pathology: (i) cell-mediated demyelination, (ii) Ab-mediated demyelination, (iii) active myelin destruction, and (iv) oligodendrogliopathy or oligodendrocyte dystrophy. In the absence of specific biomarkers, the diagnosis/prognosis of MS continues to rely on clinical history, neurological examination, and paraclinical evidences of temporal and spatial dissemination of CNS lesions. Nevertheless, the use of MRI has had a major impact, allowing the early, precise diagnosis of the disease (5, 6). Recently, it has been stated that a single diagnostic probe for MS is unlikely to serve as a general prognostic tool to cover the full clinical spectrum of MS. Therefore, the development of a number of biomarkers, each one specific for different pathophysiological mechanisms, will be important for better understanding disease pathogenesis and for future drug development in MS (7).
The most extensively studied putative self-Ags are components of normal CNS myelin [myelin basic protein (MBP); proteolipid lipoprotein, and myelin oligodendrocyte glycoprotein (MOG)], mostly posttranslationally modified proteins (8, 9). Posttranslational modifications can mask self-Ags, creating new Ags no longer recognized by the immune system. In particular, aberrant glycosylation affects the immune response and profoundly affects immune tolerance (10). Recently, IgM autoAbs to recombinant MOG (11, 12) have been reported to be predictors of clinically definite MS after a first demyelinating event. However, these results need to be confirmed (13, 14).
New candidate biomarkers in MS could emerge from multi- and interdisciplinary efforts and the application of novel unbiased discovery tools (7). With this consideration in mind, we pursued an approach to develop a specific antigenic probe that will allow identifying a population of MS patients in which presence of autoAbs, recognizing the antigenic probe, will correlate with disease activity. In fact, the efficient detection of autoAbs in a large group of MS patients guided the selection of an optimized synthetic glycopeptide Ag. This approach represents a radical departure from the conventional methods based on preselected myelin components, which displayed variable efficiency in the recognition properties for autoAbs. Whereas the traditional approach fails to detect posttranslational modifications of myelin proteins, ours overcomes these difficulties by using Abs circulating in serum and pathologically developed in an MS patient subgroup.
In light of the importance of specific sugars and conformation of epitopes involved in autoAb recognition, we have designed synthetic glycopeptides, and studied their capacity to recognize autoAbs in MS patients. The aim of this study is to develop a specific antigenic probe and a simple assay, both optimized to efficiently recognize autoAbs characteristic to a subgroup of MS patients. Selection of the optimized antigenic probe, which is well characterized chemically, was achieved by designing and screening synthetic libraries of glycopeptides. The main advantage of measuring the autoAb titer in serum through an ELISA, based on a synthetic probe, is its simplicity and the possibility to perform serial analyses.
Methods
Peptide and Glycopeptide Synthesis. The synthesis of glycopeptides requires specialized methods (15). All of the products described have been successfully synthesized by using differently glycosylated amino acids (16, 17) as building-blocks in an optimized solid-phase peptide synthesis strategy on an automatic synthesizer (APEX 396, Advanced ChemTech) following the fluorenylmethoxycarbonyl/tert-butyl strategy (see Supporting Materials and Methods, which is published as supporting information on the PNAS web site). All peptides and glycopeptides (Table 1) were obtained with a purity >98% by preparative HPLC (Waters 600), and were characterized by HPLC electrospray ionization mass spectroscopy (LCQ Advantage, ThermoFinnigan, San Jose, CA) (see Table 3, which is published as supporting information on the PNAS web site).
Table 1. Glycosylated and unglycosylated peptides synthesized as candidate specific multiple sclerosis antigenic probe.
| Name (peptide no.) | Peptide sequence |
|---|---|
| [Asn31(Glc)]hMOG(30–50) (1) | KN(Glc)ATGMEVGWYRPPFSRVVHL |
| hMOG(30–50) (1′) | KNATGMEVGWYRPPFSRVVHL |
| [Asn84(Glc)]hMBP(83–99) (2) | EN(Glc)PVVHFFKNIVTPRTP |
| hMBP(83–99) (2′) | ENPVVHFFKNIVTPRTP |
| CSF114(Glc) (3) | TPRVERN(Glc)GHSVFLAPYGWMVK |
| CSF114 (3′) | TPRVERNGHSVFLAPYGWMVK |
| CSF114(GlcAc4) (4) | TPRVERN(GlcAc4)GHSVFLAPYGWMVK |
| CSF114(GlcNAc) (5) | TPRVERN(GlcNAc)GHSVFLAPYGWMVK |
| CSF114(Glcβ4Glc) (6) | TPRVERN(Glcβ4Glc)GHSVFLAPYGWMVK |
| [Ser7(Glc)]CSF114 (7) | TPRVERS(Glc)GHSVFLAPYGWMVK |
| MBH36(Glc) (8) | RGKYTYN(Glc)GITYEGR |
| MBH36 (8′) | RGKYTYNGITYEGR |
| [Thr9]CSF114(Glc) (9) | TPRVERN(Glc)GTSVFLAPYGWMVK |
| [Thr9[CSF114 (9′) | TPRVERNGTSVFLAPYGWMVK |
| Ac-[c(Dap5, Asp10)]CSF114(Glc) (10) | Ac-TPRV-c[Dap-RN(Glc)GHD]VFLAPYGWMVK |
| Ac-[c(Dap5, Asp10)]CSF114 (10′) | Ac-TPRV-c(Dap-RNGHD)VFLAPYGWMVK |
| Scramble CSF114(Glc) (11) | LAKVSYN(Glc)FRMETRVGWHPVGP |
| Scramble CSF114(Glc) (11′) | LAKVSYNFRMETRVGWHPVGP |
c, cyclo; Dap, 2,3-diaminopropionic acid; GlcAc4, tetraacetylated glucose; Glcβ4Glc, cellobiose.
CD and NMR Studies. The far-UV CD spectra were acquired by using a model J-810 spectropolarimeter (Jasco). Four scans were acquired over 190–260 nm in 0.2-nm steps, with a bandwidth of 1 nm at 298 K. Peptides 3 and 8–11 and the corresponding unglycosylated 3′, 8′–11′ were analyzed in water and in water/hexafluoroacetone (HFA) at a 1:1 (vol:vol) ratio. NMR spectra were recorded on a DRX-600 spectrometer (Bruker, Billerica, MA). One-dimensional NMR as well as DQF-COSY, TOCSY, and NOESY experiments were run according to protocols described in ref. 17. Structural determinations and computational modeling procedures can be found in Supporting Materials and Methods.
Patients. We tested a total of 250 relapsing–remitting MS (RR-MS) patients classified according to the Poser's criteria (18). Patients were between 20 and 60 years of age (median, 34 years of age), had an expanded disability status scale (EDSS) range from 0 to 6 (median, 2.5), and a disease duration between <1 month and 30 years (median, 9 years). All patients had IgG oligoclonal bands in cerebrospinal fluid and MRI findings typical of MS.
Furthermore, in a longitudinal follow-up study, we tested 40 RR-MS patients characterized by an EDSS of <3.5 who received no therapy at the time of the sampling. As controls, we used normal blood donors (NBD) (n = 166); patients with inflammatory neurological diseases (IND) (n = 25), i.e., meningitis/encephalitis; and patients affected by other autoimmune diseases (OAD) (n = 90), i.e., rheumatoid arthritis (n = 49), and systemic lupus erythematosus (n = 41). All experiments were performed in compliance with institutional guidelines and were approved by the local hospital's ethical committees.
Detection of AutoAbs. We assessed the Ab responses against peptides and glycopeptides by solid-phase noncompetitive ELISA (SP-ELISA) with anti-human IgM or IgG Fab2-specific affinity-purified Abs conjugated to alkaline phosphatase. In parallel experiments, subclass-specific anti-IgG conjugates were used to detect the IgG Abs subclasses. Within- and between-assays coefficients of variations were <10% (see Supporting Materials and Methods).
Measurement of Ab Affinity by Competitive ELISA and Statistical Analysis. Ab affinity and Ab affinity heterogeneity were measured by following the methods published in ref. 19. The semisaturating dilution was calculated from the preliminary titration curves (absorbance, 0.7). At this dilution, Abs were preincubated with increasing Ag concentration (1 h at 25°C). Unblocked Abs were revealed by ELISA, and the antigenic probe concentration–absorbance relationship was presented graphically. The mean titers of IgM or IgG autoantibodies positively recognizing the antigenic probe in MS patients and controls were studied with the Mann–Whitney U test. The significance of P values was set at <0.001.
Immunoaffinity Columns. CSF114(Glc), dissolved in 0.1 M NaHCO3 containing 0.5 M NaCl, pH 8.3, was coupled to CNBr-Sepharose matrix. Sera from MS patients positive to CSF114(Glc) (diluted 1:10 in PBS) were loaded onto the column preequilibrated with PBS, pH 7. Adsorbed Abs were eluted by using 0.1 M glycine, pH 2.6. CSF114(Glc)-specific Abs were analyzed by UV spectroscopy. The efficiency of this immunoadsorbtion was confirmed by SP-ELISA.
Immunohistochemistry. Immunohistochemistry was performed on sections from human CNS autopsies as well as from other tissues autopsies (lung, liver, kidney, and lymph node), or biopsies (sural nerve and muscle) obtained from patients without neurological diseases as described in ref. 20. These sections were incubated overnight at 4°C with patients' sera (diluted 1:800 in PBS) or IgG/IgM purified by CSF114(Glc)-Sepharose affinity columns (1:10). As controls, we used purified IgM and biotinylated IgG from CSF114(Glc)-negative cases as well as Ig fractions obtained after depletion of CSF114(Glc)-specific Ig. Biotinylation of anti-CSF114(Glc) IgG was performed as described in ref. 21. Double immunofluorescence was performed with the neural phenotypic markers MBP (1:200) for mature oligodendrocytes, glial fibrillary acidic protein (1:500) for astrocytes, CD68 for microglia (1:100) (all from DAKO) and NG2 (1:100; Chemicon) for oligodendrocyte precursors (see Supporting Materials and Methods).
Results
Design, Synthesis, and Screening of Glycopeptides Optimally Recognizing AutoAbs in MS Patient Serum. We investigated the ability of synthetic modified peptides specifically glycosylated to mimic structural features of neo-epitopes (self-Ags possibly recognized as non-self-Ags after aberrant glycosylation) to be tested in MS. The glucosylated analogue of the immunodominant epitope of MOG, [Asn31(Glc)]hMOG (30–50) (peptide 1), which is able to detect autoAbs in MS patients (16), and the inactive unglucosylated analogue hMOG (30–50) (peptide 1′) adopted similar solution conformations. We concluded that the ability of the former molecule to detect autoAbs in MS was linked to characteristics other than conformation and that the specific binding site on MOG glycopeptide 1 was related to the N-linked glucose moiety (17).
We synthesized a series of peptide and glycopeptide sequences unrelated to MOG to characterize the autoAb recognition in serum of MS patients (Table 1). One such peptide was [Asn84(Glc)]hMBP(83–99) (peptide 2), a glucosylated partial sequence of an immunodominant epitope of MBP, perhaps the most studied MS candidate autoAg not naturally glycosylated. The second was a completely unrelated sequence, termed CSF114(Glc) (peptide 3). A structure-based design yielded CSF114(Glc) as an artificial sequence characterized by a β-turn structure, with a conformational propensity to optimally expose the sugar moiety (glucose) (22, 23).
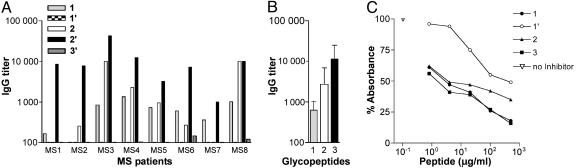
We studied the IgG Ab titer to the three glycopeptides 1–3 and to the corresponding unglycosylated sequences 1′–3′ (Table 1) by SP-ELISA in a first group of 27 patients affected by clinically definite MS (Fig. 1A). Because the sera of eight of 27 patients showed increased titers when challenged with glycopeptide CSF114(Glc), this molecule appears to be a preferred ligand for Ab binding. Although we did not detect any Ab titer to the unglycosylated peptides 1′ and 3′, in four of the above eight cases, we observed reactivity to MOG glycopeptide 1 and to MBP glycopeptide 2. It is evident, therefore, that all glycopeptides containing N-linked glucose identified high Ab titers, although CSF114(Glc) detected the highest autoAb titer in MS serum (Fig. 1B).
Fig. 1.
AutoAb recognition in RR-MS patients' sera. (A) IgG Ab titers to glycopeptides 1, 2, and 3 and to unglycosylated peptides 1′ and 3′ in eight representative RR-MS sera. (B) Mean IgG Ab titers reported in A to glycopeptides 1, 2, and 3.(C) Inhibition curves of Abs binding to glycopeptide 1 with glycopeptides 1, 2, and 3 and unglycosylated peptide 1′ in a competitive ELISA. The results are expressed as the percentage of absorbance of a representative RR-MS serum of the five of eight positive to glycopeptide 1 (ordinate axis). The concentrations of the peptides used as inhibitors are on the abscissa axis.
Considering that SP-ELISA reflects essentially the relative affinity, which depends on the exposure of the epitope in the solid phase conditions of the assay, we also investigated the absolute Ab affinity in a competitive ELISA (19, 24) based on inhibition of autoAbs in solution. In a set of three MS positive sera, glycopeptides 1–3 inhibited the binding of autoAbs to glycopeptide 1, giving rise to similar inhibition curves (IC50 corresponding to 20–30 μg/ml, i.e., 10-7 M). In contrast, unglycosylated peptide 1′ showed no inhibitory activity. The data of a representative serum (Fig. 1C) show that the three glycopeptides exhibited superimposable affinity in competitive ELISA, despite the differences of apparent affinity detected in SP-ELISA (Fig. 1B). This finding indicated that the three glycopeptides had an identical epitope, which should contain an Asn(Glc) residue.
Epitope Characterization. Role of the N-glucosyl moiety. By testing a small focused library of CSF114 analogues characterized by glycoamino acid diversity (Table 1, compounds 4–7), we were able to definitively determine that the minimal epitope had to contain an Asn(Glc) moiety. In fact, glycopeptides lacking Asn(Glc) displayed negligible inhibitory activity in competitive ELISA and failed to detect IgG autoAbs in SP-ELISA (data not shown).
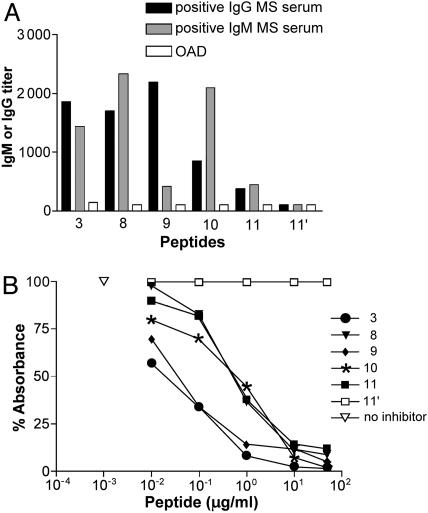
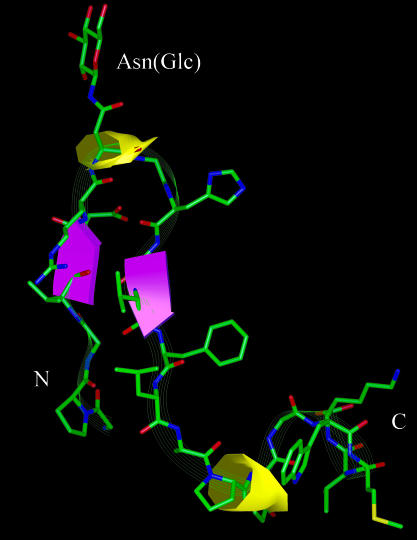
Role of the glycopeptide conformation. A second focused library of glycopeptides based on structural diversity (Table 1, compounds 8–11) allowed us to determine the importance of the type I′ β-turn structure (25) for exposure of the minimal epitope (N-linked glucose). Glycopeptides 8–10 and CSF114(Glc), all possessing the β-hairpin motif, revealed increased IgM and/or IgG titers in SP-ELISA (Fig. 2A). As expected, these glycopeptides, which contained the minimal epitope Asn(Glc), displayed affinity for MS autoAbs comparable to CSF114(Glc) (peptide 3) in a competitive ELISA (Fig. 2B). Differently, glycopeptide 11, an unstructured peptide scrambled in its amino acid sequence relative to CSF114(Glc), did not recognize autoAbs in SP-ELISA because it was unable to expose the epitope Asn(Glc) in the solid-phase conditions of the assay (Fig. 2A). Structural studies by CD, one- and two-dimensional proton NMR, and molecular modeling techniques demonstrated that CSF114(Glc) possesses a β-hairpin structure, with the minimal epitope Asn(Glc) at position i + 1 of a type I′ β-turn nicely exposed to Ab recognition (Fig. 3). It appears therefore that the β-turn conformation of CSF114(Glc) is fundamental for a correct exposure of the epitope containing Asn(Glc).
Fig. 2.
AutoAb recognition in MS patients' sera to structurally diverse glycopeptides. (A) IgM and IgG Ab titer (ordinates axis) to the glycopeptides of the focused library based on structural diversity (peptides 8–11) and to unglycosylated peptide 11′ compared with glycopeptide 3 of two representative RR-MS sera (IgM/IgG Abs) and one representative OAD serum. (B) Inhibition curves of anti-CSF114(Glc) Abs with glycopeptides 8–11 and with unglycosylated peptide 11′, in comparison with CSF114(Glc) in a competitive ELISA. The results are expressed as the percentage of absorbance of a representative RR-MS serum (ordinate axis). The concentrations of the peptides used as inhibitors are on the abscissa axis.
Fig. 3.
Calculated structures of CSF114(Glc). Shown is a ribbon diagram of the lowest energy conformer of 200 calculated structures of CSF114(Glc) derived from NMR data. The miniβ-sheet involving residues 4–5 and 10–11 is depicted in violet. The turn motifs encompassing residues 6–9 and 14–17 are shown in yellow. Heavy atoms are shown with the following color code: green, carbon; red, oxygen; blue, nitrogen; and yellow, sulfur. Hydrogen atoms are not shown for clarity. The NH2 and COOH termini are labeled N and C, respectively.
Validation Study in a Clinically Relevant Number of MS Patients: Cross-Sectional and Long-Term Analysis. We extended the analysis of Abs to CSF114(Glc) to a larger series of MS patients and controls (531 sera in total). In the RR-MS population examined (n = 250), we detected increased mean levels of IgM Ab titers to CSF114(Glc) (Table 2). The mean titers of anti-CSF114(Glc) IgM Abs were increased in MS (mean titer, 943 ± 488) versus the titers observed in NBD (488 ± 504; P < 0.001, Mann–Whitney U test). The IgM titers observed in OAD were significantly lower than in MS (467 ± 366, P < 0.001). Thus, the results in OAD were not different from NBD. On the contrary, we detected increased anti-CSF114(Glc) IgM Abs in patients affected by meningitis and encephalitis (IND) in acute phase who were used as controls of a condition associated with brain inflammation and myelin destruction (26). Therefore, anti-CSF114(Glc) IgM Abs have a potential important diagnostic value in MS, provided that a clinical evaluation exclude the occurrence of acute IND.
Table 2. Anti-CSF114(Glc) IgM titers, but not IgG, in RR-MS patients are significantly increased compared with NBD and OAD.
| RR-MS | OAD | NBD | IND | |
|---|---|---|---|---|
| IgM titers | 943 ± 488* | 467 ± 366 | 488 ± 504 | 999 ± 674* |
| IgM titers above cut-off | 51 (21) | 0 (0) | 10 (6) | 7 (27) |
| IgG titers | 816 ± 814 | 400 ± 401 | 866 ± 872 | 854 ± 801 |
| IgG titers above cut-off | 65 (26) | 3 (3) | 36 (21) | 9 (36) |
Comparison of anti-CSF114(Glc) IgM and IgG mean titers in RR-MS patients (n = 250), OAD (n = 90), NBD (n = 166), and IND (n = 25). Values for IgM and IgG titers are means ± SD. IgM cut-off was set at the mean ± 2 SD of NBD. IgG cut-off was set at 1,000. Values in parentheses indicate percentages. *, P < 0.001 vs. NBD by Mann-Whitney U test.
We tested also anti-CSF114(Glc) IgG responses (Table 2). Importantly, we found a very low mean Ab titer in OAD (IgG mean titer, 400 ± 401) compared with the mean Ab titer in RR-MS, NBD, and IND, which was in the 800 range. All of the IgG responses corresponded to the IgG2 subclass (data not shown).
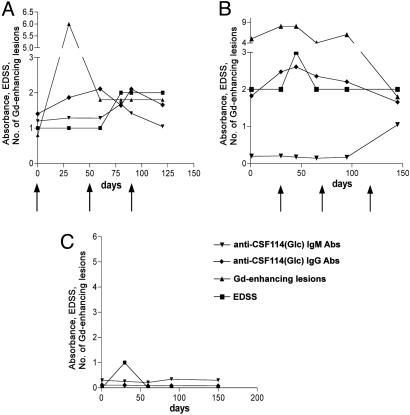
We explored the potential of CSF114(Glc) to monitor disease evolution. We followed longitudinally for up to 6 months a group of 40 untreated RR-MS patients with an EDSS of <3.5. The choice to enroll patients in an untreated period was aimed at avoiding the influence of therapies on autoAb levels. During the study, we recorded the clinical activity, monthly MRI scans, and the anti-CSF114(Glc) IgM and IgG Ab titers. As an example, we present the course of the Ab response to CSF114(Glc) in three representative MS patients (Fig. 4), two of which (Fig. 4 A and B) presented clinical activity (Fig. 4, relapse at arrows) and Gd-enhancing positive brain MRI lesions. For comparison, we present also an inactive patient (Fig. 4C). The observed Ab response parallels the occurrence of inflammatory brain lesions detected by MRI and the clinical activity. In total, we detected Ab response to CSF114(Glc) in 16 of 40 (40%) patients showing Gd-positive MRI lesions.
Fig. 4.
Longitudinal study of three representative RR-MS patients. Course of Ab response (IgM and IgG absorbance) to CSF114(Glc), EDSS, and number of Gd-enhancing MRI brain lesions in three representative MS patients. (A and B) Two representative MS patients belonging to the longitudinal study group presenting high anti-CSF114(Glc) Abs, clinical activity (relapse at arrows), and brain MRI lesions. (C) An inactive patient.
Isolation and CNS Specificity of Anti-CSF114(Glc) AutoAbs from MS Patients. Specific anti-CSF114(Glc) autoAbs were isolated from MS patients' sera by using an immunoaffinity column that anchored CSF114(Glc) to Sepharose. After loading sera, adsorbed IgM and IgG autoAbs were eluted, which fully recovered their activity, as determined by SP-ELISA. This experiment indicates that CSF114(Glc) affinity columns provide a convenient one-step procedure to isolate autoAbs from CSF114(Glc)-positive MS sera.
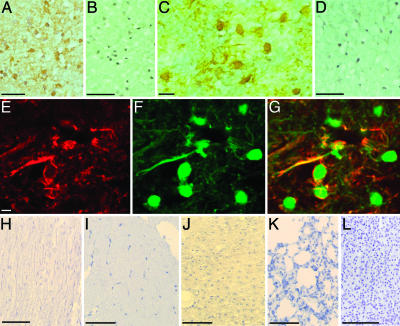
To assess whether anti-CSF114(Glc) autoAbs recognized CNS structures in situ, we tested sera from patients with either high Ab titers (10 MS cases) or no reactivity to CSF114(Glc) (5 MS, 10 healthy subjects, and 5 patients affected by other neurological diseases) by immunohistochemistry on sections from nonpathological human CNS specimens (20). All 10 CSF114(Glc)-positive sera tested showed diffuse IgM immunostaining of CNS white matter, particularly on myelin sheaths, and glial cells morphologically resembling oligodendrocytes (Fig. 5A). Immunostaining for IgM from all 20 CSF114(Glc)-negative cases gave no detectable signals in CNS sections (Fig. 5B). We then explored which Ab fraction in CSF114(Glc)-positive sera was responsible for CNS immunostaining. For this purpose, we used CSF114(Glc)-specific IgG or IgM affinity-purified Abs and IgG biotinylated isolated from two CSF114(Glc)-positive MS sera. The immunostaining with CSF114(Glc)-specific IgG or IgM (Fig. 5C) was comparable with that of the respective sera, staining myelin, and small round glial cells in CNS white matter. At variance, Ig fractions depleted of anti-CSF114(Glc) autoAbs gave no CNS immunoreactivity (Fig. 5D). We demonstrated the precise cellular distribution of the CNS Ags recognized by anti-CSF114(Glc) autoAbs by double immunofluorescence experiments. CSF114(Glc)-specific, biotinylated IgG and IgM colocalized with MBP, an oligodendroglial phenotypic marker on myelin sheaths, and at the surface of mature oligodendrocytes (Fig. 5 E–G). We did not observe any positive signal for anti-CSF114(Glc)-specific biotinylated IgG in non-CNS human tissues, which included sural nerve, muscle, liver, lung, and kidney (Fig. 5 H–L), respectively, thus confirming the CNS specificity of anti-CSF114(Glc) Abs.
Fig. 5.
CNS specificity of anti-CSF114(Glc) autoAbs. (A and B) Immunoperoxidase reaction for IgM onto CNS sections reveals diffuse staining in the white matter on myelin and on small glial cells, morphologically resembling oligodendrocytes, after incubation of CSF114(Glc)-positive MS serum (A) but not of CSF114(Glc)-negative serum (B). (C) Myelin and glial cells of CNS white matter were also immunostained by CSF114(Glc)-specific anti-CSF114(Glc) IgG Abs affinity-purified from an MS patient. (D) After depletion of anti-CSF114(Glc)-specific Ig, no detectable staining was observed for IgM. (E and F) The double immunofluorescence with biotinylated anti-CSF114(Glc) IgG Abs from the same MS patient [Texas red (E)] and with the oligodendroglial cell marker MBP [fluorescein (F)] showed that Ags recognized by anti-CSF114(Glc)-specific IgG Abs were localized on myelin sheaths and on the surface of oligodendrocytes. (G) Merged image of E and F. (H–L) No positive signals for anti-CSF114(Glc)-specific IgG were detected in non-CNS human tissues, which included sural nerve (H), muscle (I), liver (J), lung (K), and kidney (L). (Bars, 80 μm.)
Discussion
This study describes a synthetic glycopeptide termed CSF114(Glc), which functions as an efficient antigenic probe to detect, isolate, and characterize Abs as biomarkers of MS. CSF114(Glc) is a simple, reliable, and efficient tool, which demonstrates that an aberrant N-glucosylation is involved in autoAb recognition in MS.
The guiding principle for the selection of this antigenic probe from focused peptide libraries of putative Ags was the efficient detection of autoAbs present in patients' sera. Within these libraries, we designed peptides to bear small variations of amino acid sequence or sugar moiety to rationally achieve optimal conformation of the epitope recognized by Abs.
This approach represents a radical departure from the conventional one based on preselected myelin components, i.e., biochemically isolated and/or recombinant molecules that display variable efficiency in the recognition properties for autoAbs.
A significant advantage offered by the synthetic approach is its ability to overcome the difficulties inherent to the traditional ones, which may not detect posttranslational modifications, such as glycosylation of myelin proteins that could be affected by virus and/or bacterial infections or by defects in the glycosylation pathway.
We demonstrated the value of CSF114(Glc) at several levels. At the molecular level, the main feature of the synthetic Ag lies in the glycopeptide fragment, which, by virtue of the peculiar type I′ β-turn structure, optimally exposes the minimal epitope Asn(Glc) to autoAbs, recognizing them with high affinity and great specificity. At the cellular level, we proved the ability of CSF114(Glc)-specific Abs to recognize myelin and oligodendrocyte autoAgs in brain tissue. In parallel, we did not observe similar Ags in nonrelevant human tissues, such as sural nerve, muscle, liver, lung, and kidney, thus suggesting that the Ag is brain-specific.
Finally, at the clinical level, CSF114(Glc) detected autoAbs in MS serum by a simple SP-ELISA. We clearly demonstrated the presence of high titers of anti-CSF114(Glc) IgM Abs in 21% (51/250) of the examined population of RR-MS patients (Table 2). The specificity was highest for IgM autoAbs as shown by the higher percentage of MS patients recognized by our Ag relative to NBD (P = 0.0001) or OAD (rheumatoid arthritis and systemic lupus erythematosus), in which the Ab titer was negligible (P < 0.001). In accordance with our results, recent reports have greatly emphasized the relevance of IgM for the autoAb response in MS (11, 27). The biological importance of IgM binding to different glycosylated epitopes was further confirmed in experimental models of demyelination when these Abs are synthesized within the CNS (28).
In our view, the IgG titer as detected by CSF114(Glc) appears to be unspecific because it detects a “noise” level in all groups examined except OAD. Perhaps this noise originates from carbohydrate Ags, which are important components of both innate and adaptive antimicrobial immunity. The high rate of positive results among NBD for IgG autoAbs is in line with recently reported data indicating that specific autoAbs precede by several years the onset of autoimmune diseases (29). Consequently, NBD's single serum samples are a less significant control as compared with clinically characterized patients affected by other autoimmune conditions in which anti-CSF114(Glc) IgG are virtually absent.
Because many microbial oligosaccharide structures are also expressed in humans, immune responses directed to these structures commonly occur as memory-protective IgG responses in healthy subjects after common infection. The predominant IgM and IgG2 response detected in the present study seems to be reminiscent of a T cell-independent B activation that is known to be stimulated by many nonproteic Ags (30).
Moreover, the presence of anti-CSF114(Glc) Abs in patients affected by meningitis and encephalitis (IND) does not affect the specificity of our tool, but, on the contrary, this result strongly supports the usefulness of our test. In fact, it is possible that the detected autoAbs are a measurement of a systemic immune response during a neuroinflammatory condition, monitoring major tissue inflammation, demyelination, and necrosis processes (26). Because sera from IND were collected in the acute phase, the high anti-CSF114(Glc) Ig titer will decline in analogy to that occurring for other anti-myelin autoAbs (31).
Although CSF114(Glc) as an Ag was less discriminating toward IgG response, we importantly demonstrated in the longitudinal analysis of MS patients that the combined IgM and IgG Ab titer is instrumental in monitoring disease activity. We followed for up to 6 months a consistent group of untreated MS patients (n = 40) with a clearly defined disease by using the ELISA assay on sera with CSF114(Glc) as an Ag. The IgM and IgG Ab response to CSF114(Glc) recorded at regular intervals paralleled the occurrence of MRI lesions and disease progression in ≈40% of the patient population (16/40). These results showed the potent value of IgM and IgG Ab titers in detecting disease activity. In this instance, IgG determination has a high prognostic value.
Thus, the synthetic glycopeptide CSF114(Glc) appears to delineate a subgroup of RR-MS patients in which disease activity, MRI lesions, and, therefore, demyelination is associated with an increased titer of Abs to CSF114(Glc). Moreover, because increased responses to this synthetic Ag ran concurrently with relapsing episodes, the assay may assume a predictive value for disease progression.
Up to now only low-affinity anti-myelin autoAbs have been detected in sera of MS patients. Although anti-myelin proteins (MBP or MOG) Abs were reported as possible diagnostic tools for prediction of development of clinically established MS after the appearance of the first clinical symptoms (11), no correlation with disease activity and disability progression has been demonstrated (7). Moreover, other authors found by techniques other than ELISA (radio-binding assay and Western blot analysis) a similar frequency of low-affinity anti-MOG Abs in sera of MS patients and healthy subjects (13, 14).
Considering the pathophysiological heterogeneity of MS, CSF114(Glc) could help to characterize the MS subtype associated with Ab-mediated demyelination, having an important impact in diagnosis and prognosis of the disease as well as on the selection of therapy in an effort to provide individualized treatment (32).
Therefore, our results demonstrate that CSF114(Glc) can be used in a simple ELISA test as the first MS antigenic probe for detecting specific autoAbs that can serve as reliable biomarkers for the practical evaluation of the disease activity in a subpopulation of MS patients.
Supplementary Material
Acknowledgments
This paper is dedicated to our friend and colleague Marco Vergelli (1963–2001). This work was funded in part by Project Multiple Sclerosis, 1995–1999 (Istituto Superiore di Sanità); Project Regione Campania L41/94, 1999; Progetti di Ricerca di Rilevante Interesse Nazionale 2002 (Ministero dell'Istruzione, dell'Università e della Ricerca); and Fondazione Ente Cassa di Risparmio di Firenze of Italy.
Author contributions: F.L., P.R., M.C., and A.M.P. designed research; B. Mulinacci, A.C., G.S., B. Mazzanti, A.M.D., E.N., M.P., L.L., M.C.A., E.P., M.C.P.-C., F.N., and G.B. performed research; F.L., A.C., B.B., L.B., M.C., P.R., and A.M.P. analyzed data; F.L., P.R., and A.M.P. wrote the paper; and A.M.P. coordinated the multidisciplinary group.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Ag, antigen; IND, inflammatory demyelinating diseases; MBP, myelin basic protein; MOG, myelin oligodendrocyte glycoprotein; MS, multiple sclerosis; NBD, normal blood donors; OAD, other autoimmune diseases; RR-MS, relapsing-remitting MS; SP-ELISA, solid-phase not competitive indirect ELISA; EDSS, expanded disability status scale.
References
- 1.Thompson, A. J. (1996) Clin. Immunother. 5, 1-11. [Google Scholar]
- 2.Whetten-Goldstein, K., Sloan, F. A., Goldstein, L. B. & Kulas, E. D. (1998) Mult. Scler. 4, 419-425. [DOI] [PubMed] [Google Scholar]
- 3.Miltenburger, C. & Kobelt, G. (2002) Clin. Neurol. Neurosurg. 104, 272-275. [DOI] [PubMed] [Google Scholar]
- 4.Lucchinetti, C., Bruck, W., Parisi, J., Scheithauer, B., Rodriguez, M. & Lassmann, H. (2000) Ann. Neurol. 47, 707-717. [DOI] [PubMed] [Google Scholar]
- 5.Hafler, D. A. (2004) J. Clin. Invest. 113, 788-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giovannoni, G., Kieseier, B. & Hartung, H. P. (1998) J. Neurol. Neurosurg. Psychiatry 64, S31-S36. [PubMed] [Google Scholar]
- 7.Bielekova, B. & Martin, R. (2004) Brain 127, 1463-1478. [DOI] [PubMed] [Google Scholar]
- 8.de Seze, J., Dubucquoi, S., Lefranc, D., Virecoulon, F., Nuez, I., Dutoit, V., Vermersch, P. & Prin, L. (2001) J. Neuroimmunol. 117, 149-155. [DOI] [PubMed] [Google Scholar]
- 9.Kim, J. K., Mastronardi, F. G., Wood, D. D., Lubman, D. M., Zand, R. & Moscarello, M. A. (2003) Mol. Cell. Proteomics 2, 453-462. [DOI] [PubMed] [Google Scholar]
- 10.Doyle, H. A. & Mamula, M. J. (2001) Trends Immunol. 22, 443-449. [DOI] [PubMed] [Google Scholar]
- 11.Berger, T., Rubner, P., Schautzer, F., Egg, R., Ulmer, H., Mayringer, I., Dilitz, E., Deisenhammer, F. & Reindl, M. (2003) N. Engl. J. Med. 349, 139-145. [DOI] [PubMed] [Google Scholar]
- 12.Gaertner, S., de Graaf, K. L., Greve, B. & Weissert, R. (2004) Neurology 63, 2381-2383. [DOI] [PubMed] [Google Scholar]
- 13.Mantegazza, R., Cristaldini, P., Bernasconi, P., Baggi, F., Pedotti, R., Piccini, I., Mascoli, N., Mantia, L. L., Antozzi, C., Simoncini, O., et al. (2004) Int. Immunol. 16, 559-565. [DOI] [PubMed] [Google Scholar]
- 14.Lampasona, V., Franciotta, D., Furlan, R., Zanaboni, S., Fazio, R., Bonifacio, E., Comi, G. & Martino, G. (2004) Neurology 62, 2092-2094. [DOI] [PubMed] [Google Scholar]
- 15.Kunz, H. & Schultz, M. (1997) in Glycopeptides and Related Compounds: Synthesis, Analysis, and Applications, eds. Large, D. G. & Warren, C. D. (Dekker, New York), pp. 23-78.
- 16.Mazzucco, S., Matà, S., Vergelli, M., Fioresi, R., Nardi, E., Mazzanti, B., Chelli, M., Lolli, F., Ginanneschi, M., Pinto, F., et al. (1999) Bioorg. Med. Chem. Lett. 9, 167-172. [DOI] [PubMed] [Google Scholar]
- 17.Carotenuto, A., D'Ursi, A. M., Nardi, E., Papini, A. M. & Rovero, P. (2001) J. Med. Chem. 44, 2378-2381. [DOI] [PubMed] [Google Scholar]
- 18.Poser, C. M., Paty, D. W., Scheinberg, L., McDonald, W. I., Davis, F. A., Ebers, G. C., Johnson, K. P., Sibley, W. A., Silberberg, D. H. & Tourtellotte, W. W. (1983) Ann. Neurol. 13, 227-231. [DOI] [PubMed] [Google Scholar]
- 19.Rath, S., Stanley, C. M. & Steward, M. W. (1988) J. Immunol. Methods 106, 245-249. [DOI] [PubMed] [Google Scholar]
- 20.Valdo, P., Stegagno, C., Mazzucco, S., Zuliani, E., Zanusso, G., Moretto, G., Raine, C. S. & Bonetti, B. (2002) J. Neuropathol. Exp. Neurol. 61, 91-98. [DOI] [PubMed] [Google Scholar]
- 21.Harlow, E. & Lane, D. (1988) in Antibodies: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY), p. 341.
- 22.Papini, A. M., Rovero, P., Chelli, M. & Lolli, F. (2001) PCT Int. Patent Appl. WO 03/000733 A2; (2003) Chem. Abstr. 138, 56249. [Google Scholar]
- 23.Papini, A. M. (2005) Nat. Med. 11, 13. [DOI] [PubMed] [Google Scholar]
- 24.Loomans, E. E., Gribnau, T. C., Bloemers, H. P. & Schielen, W. J. (1998) J. Immunol. Methods 221, 119-130. [DOI] [PubMed] [Google Scholar]
- 25.Ramirez-Alvarado, M., Blanco, F. J., Niemann, H. & Serrano, L. (1997) J. Mol. Biol. 273, 898-912. [DOI] [PubMed] [Google Scholar]
- 26.Lolli, F., Fredrikson, S., Kam-Hansen, S. & Link, H. (1987) Ann. Neurol. 22, 67-71. [DOI] [PubMed] [Google Scholar]
- 27.Lucchinetti, C. F., Mandler, R. N., McGavern, D., Bruck, W., Gleich, G., Ransohoff, R. M., Trebst, C., Weinshenker, B., Wingerchuk, D., Parisi, J. E., et al. (2002) Brain 125, 1450-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenbluth, J., Schiff, R., Liang, W. L. & Dou, W. (2003) J. Neurocytol. 32, 265-276. [DOI] [PubMed] [Google Scholar]
- 29.Nielen, M. M., van Schaardenburg, D., Reesink, H. W., van de Stadt, R. J., van der Horst-Bruinsma, I. E., de Koning, M. H., Habibuw, M. R., Vandenbroucke, J. P. & Dijkmans, B. A. (2004) Arthritis Rheum. 50, 380-386. [DOI] [PubMed] [Google Scholar]
- 30.Barrett, D. J. & Ayoub, E. M. (1986) Clin. Exp. Immunol. 63, 127-134. [PMC free article] [PubMed] [Google Scholar]
- 31.Reindl, M., Linington, C., Brehm, U., Egg, R., Dilitz, E., Deisenhammer, F., Poewe, W. & Berger, T. (1999) Brain 122, 2047-2056. [DOI] [PubMed] [Google Scholar]
- 32.Waegemans, T. (2004) Biomed. Pharmacother. 58, 282-285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.