Abstract
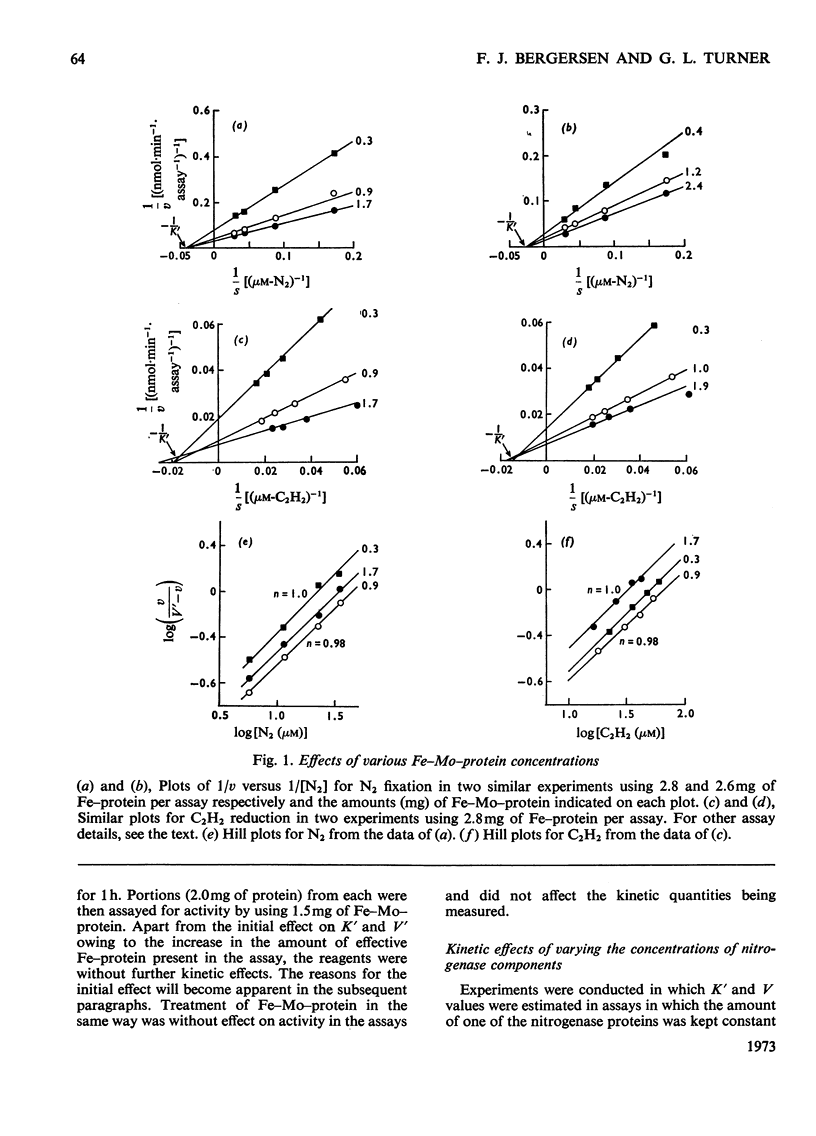
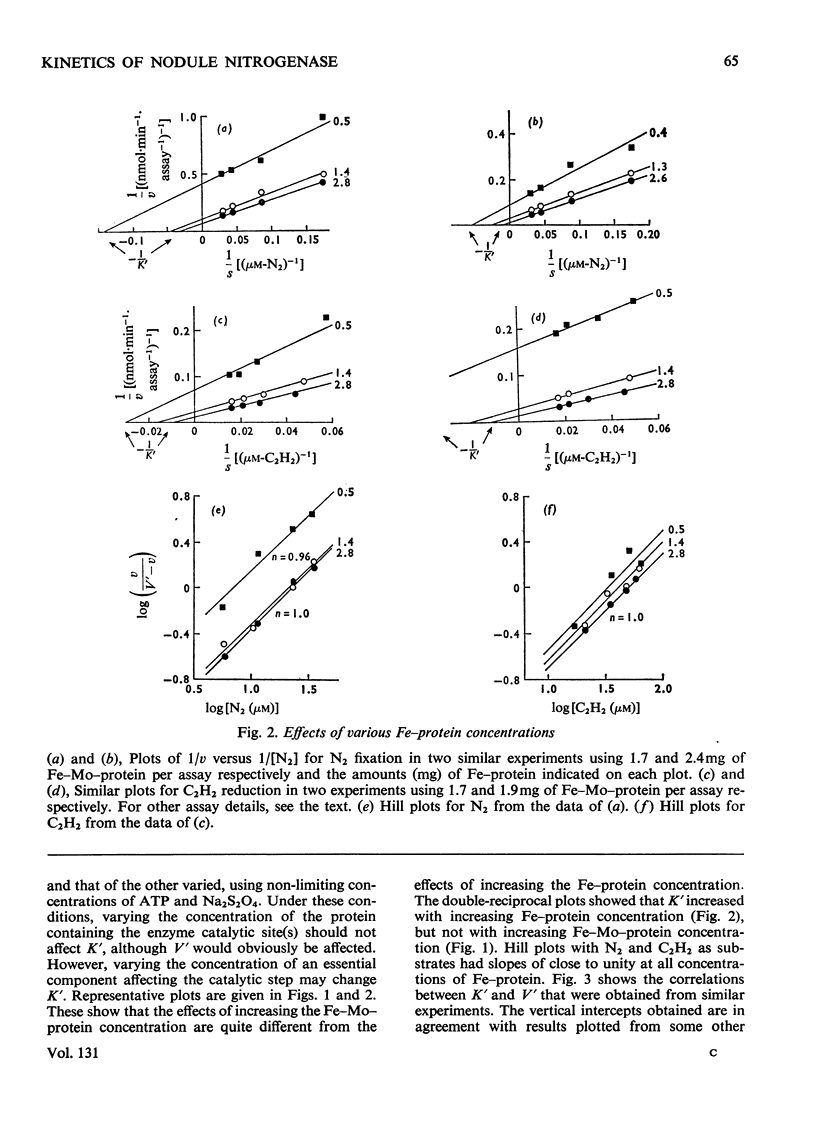
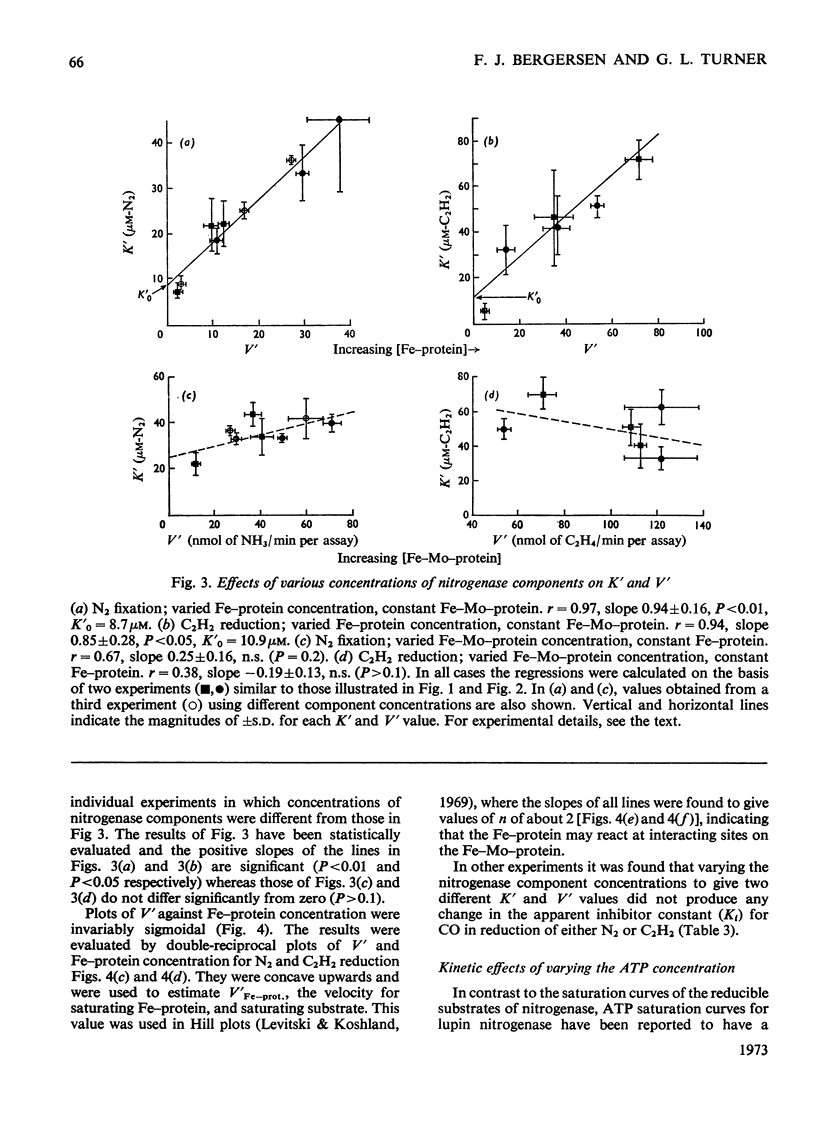
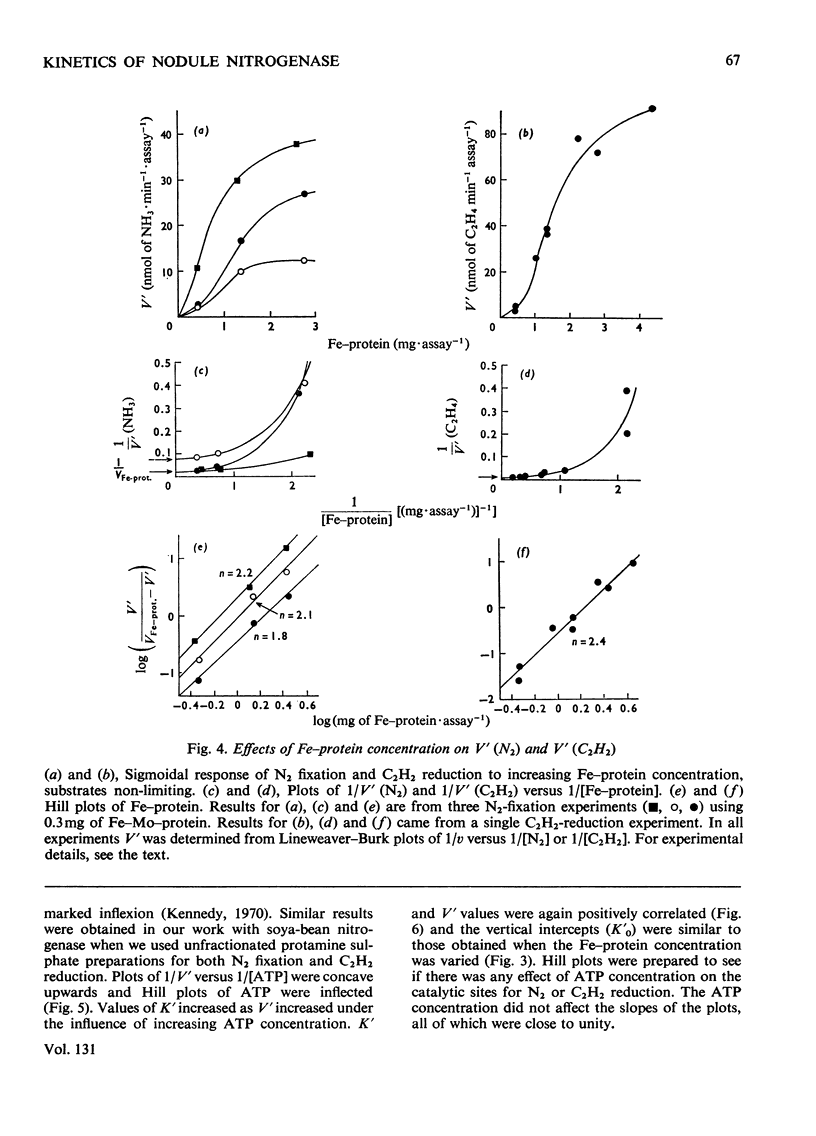
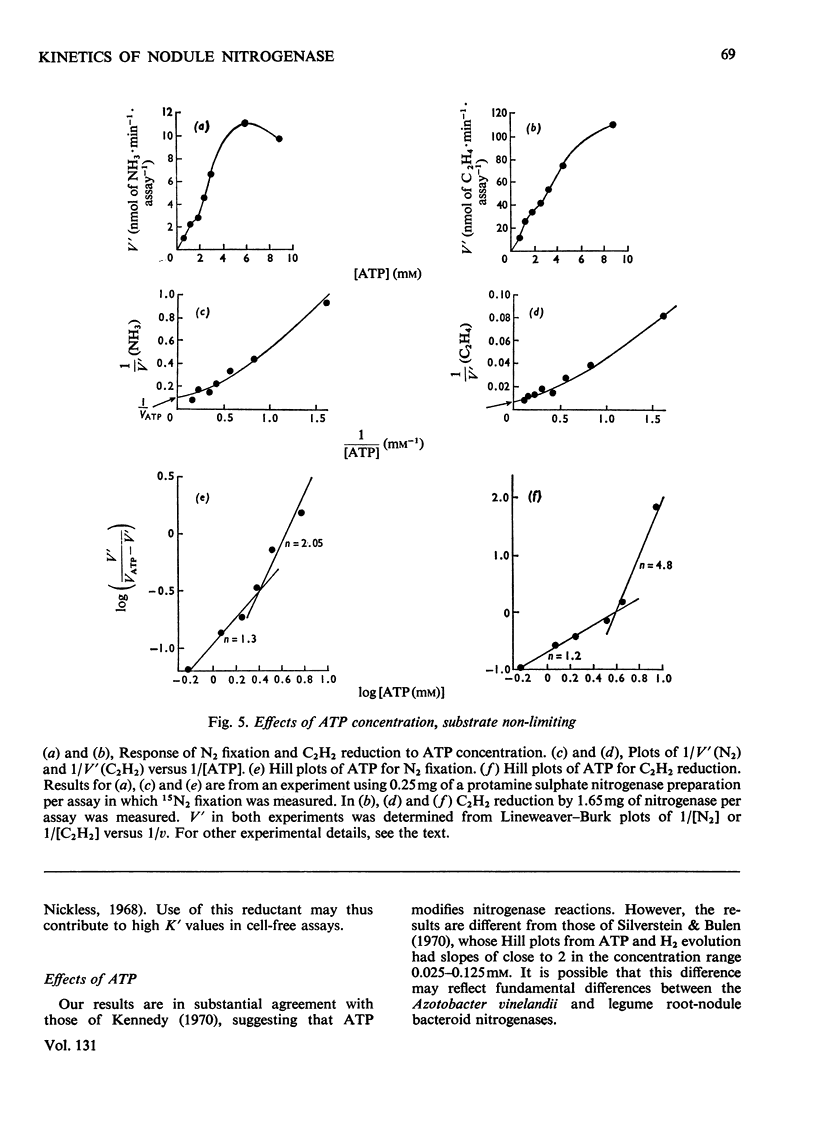
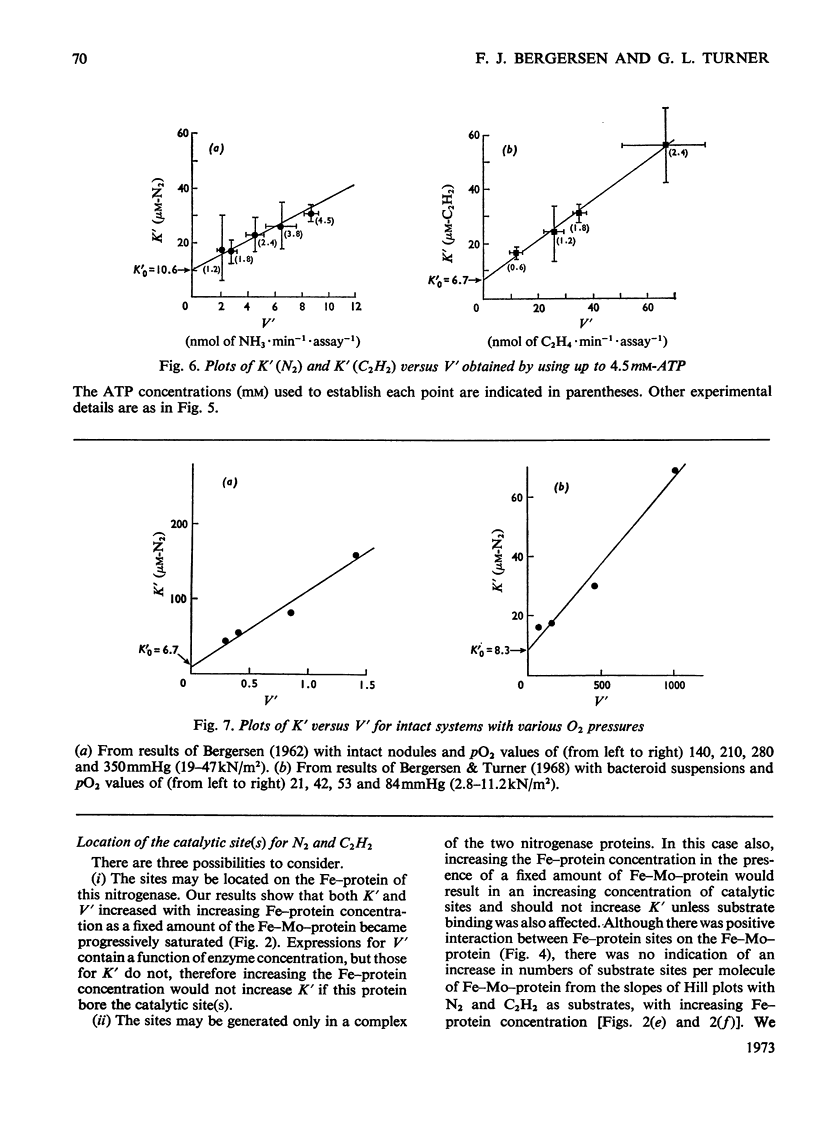
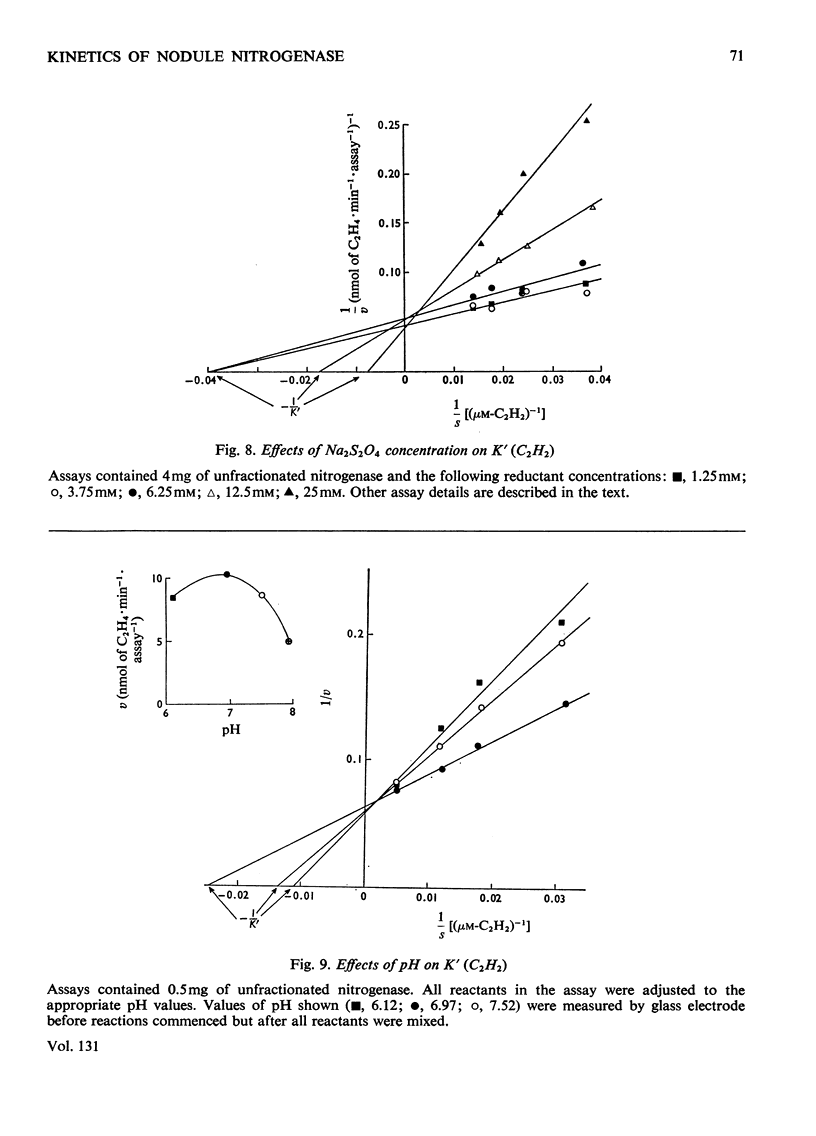
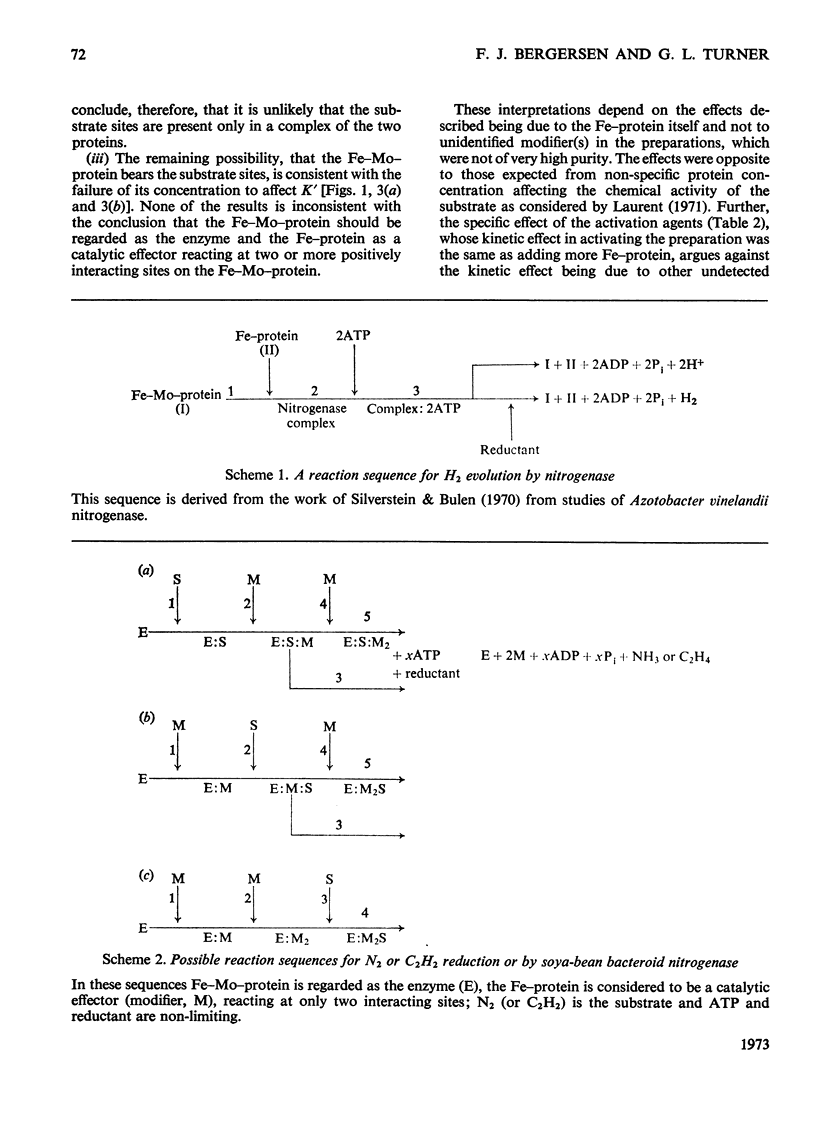
The apparent Michaelis constants [K′(N2) and K′(C2H2)] and the corresponding apparent maximum velocity values (V′) for soya-bean bacteroid nitrogenase increased concomitantly in response to increases in nitrogenase Fe–protein concentration and ATP concentration in cell-free assays and in response to O2 pressure in intact nodules and bacteroid suspensions. K′(C2H2) in cell-free assays was also affected by pH and by Na2S2O4 concentration. Nitrogenase Fe–protein behaved as a catalytic effector reacting at interacting sites on the nitrogenase Fe–Mo–protein. The results indicated that the Fe–Mo–protein probably bears the catalytic sites for N2 and C2H2 reduction. It is concluded that reduction of N2 or C2H2 by this nitrogenase involves a reaction mechanism with a sequence of unknown order. The sequence in which substrate, enzyme, effector, ATP and reductant react determines which of the various rate-constants are involved in the apparent Michaelis constant, whose true kinetic meaning was thus unresolved.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergersen F. J. Nitrogen fixation in legume root nodules: biochemical studies with soybean. Proc R Soc Lond B Biol Sci. 1969 Apr 1;172(1029):401–416. doi: 10.1098/rspb.1969.0029. [DOI] [PubMed] [Google Scholar]
- Bergersen F. J., Turner G. L. Gel filtration of nitrogenase from soybean root-nodule bacteroids. Biochim Biophys Acta. 1970 Jul 27;214(1):28–36. doi: 10.1016/0005-2795(70)90066-8. [DOI] [PubMed] [Google Scholar]
- Bui P. T., Mortenson L. E. Mechanism of the enzymic reduction of N2: the binding of adenosine 5'-triphosphate and cyanide to the N2-reducing system. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1021–1027. doi: 10.1073/pnas.61.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton H., Morris J. A., Ward M. A., Mortenson L. E. Purification and some properties of molybdoferredoxin, a component of nitrogenase from Clostridium pasteurianum. Biochemistry. 1971 May 25;10(11):2066–2072. doi: 10.1021/bi00787a016. [DOI] [PubMed] [Google Scholar]
- Dua R. D., Burris R. H. Studies of cold lability and purification of a nitrogen-activating enzyme. Biochim Biophys Acta. 1965 Jun 22;99(3):504–510. doi: 10.1016/s0926-6593(65)80203-x. [DOI] [PubMed] [Google Scholar]
- Evans M. C.W., Telfer Alison, Cammack R., Smith R. V. EPR studies of nitrogenase: ATP dependent oxidation of fraction 1 protein by cyanide. FEBS Lett. 1971 Jul 1;15(4):317–319. doi: 10.1016/0014-5793(71)80647-6. [DOI] [PubMed] [Google Scholar]
- Hardy R. W., Holsten R. D., Jackson E. K., Burns R. C. The acetylene-ethylene assay for n(2) fixation: laboratory and field evaluation. Plant Physiol. 1968 Aug;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M., Lang G. Evidence from Mossbauer spectroscopy for the role of iron in nitrogen fixation. Biochim Biophys Acta. 1970 Nov 3;223(1):86–104. doi: 10.1016/0005-2728(70)90134-9. [DOI] [PubMed] [Google Scholar]
- Kennedy I. R. Kinetics of acetylene and CN- reduction by the N2-fixing system of Rhizobium lupini. Biochim Biophys Acta. 1970 Oct 27;222(1):135–144. doi: 10.1016/0304-4165(70)90358-2. [DOI] [PubMed] [Google Scholar]
- Klucas R. V., Koch B., Russell S. A., Evans H. J. Purification and Some Properties of the Nitrogenase From Soybean (Glycine max Merr.) Nodules. Plant Physiol. 1968 Dec;43(12):1906–1912. doi: 10.1104/pp.43.12.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch B., Evans H. J., Russell S. Properties of the nitrogenase system in cell-free extracts of bacteroids from soybean root nodules. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1343–1350. doi: 10.1073/pnas.58.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laurent T. C. Enzyme reactions in polymer media. Eur J Biochem. 1971 Aug 25;21(4):498–506. doi: 10.1111/j.1432-1033.1971.tb01495.x. [DOI] [PubMed] [Google Scholar]
- Levitzki A., Koshland D. E., Jr Negative cooperativity in regulatory enzymes. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1121–1128. doi: 10.1073/pnas.62.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein R., Bulen W. A. Kinetic studies of the nitrogense-catalyzed hydrogen volution and nitrogen reduction reactions. Biochemistry. 1970 Sep 15;9(19):3809–3815. doi: 10.1021/bi00821a021. [DOI] [PubMed] [Google Scholar]
- Turner G. L., Bergersen F. J. The relationship between nitrogen fixation and the production of HD from D2 by cell-free extracts of soya-bean nodule bacteroids. Biochem J. 1969 Nov;115(3):529–535. doi: 10.1042/bj1150529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]


