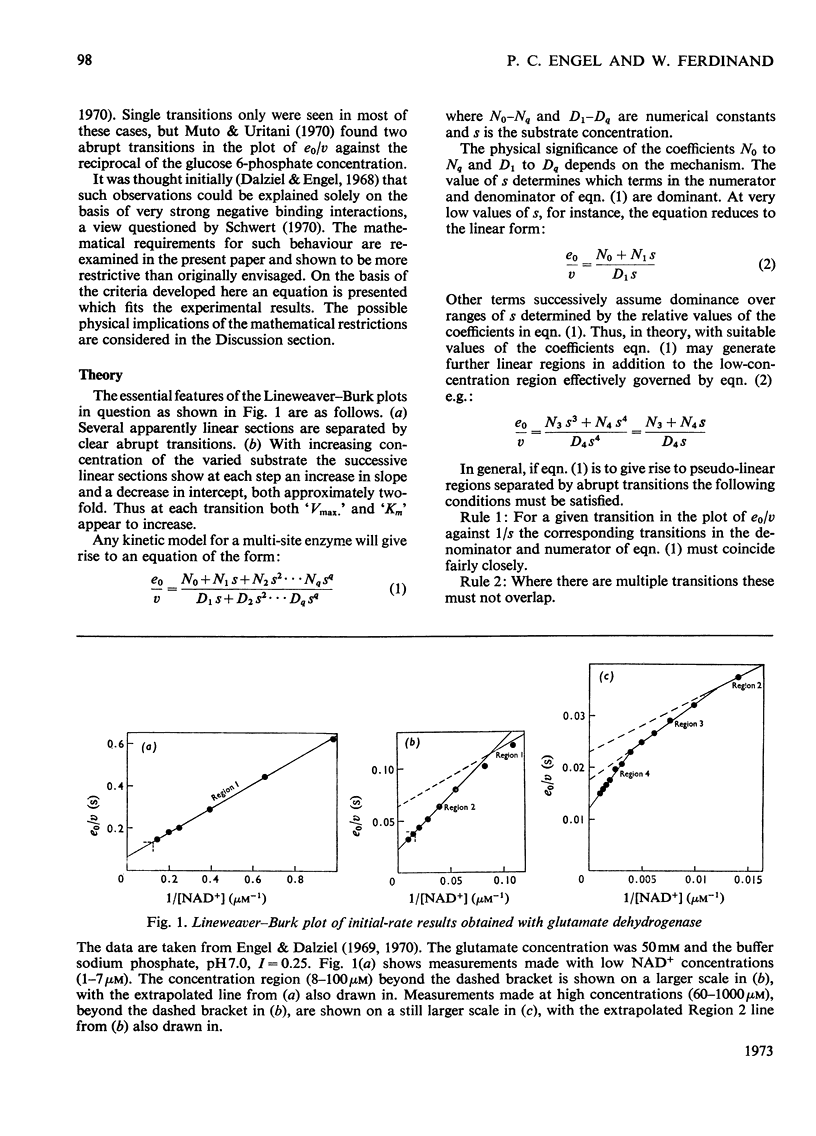
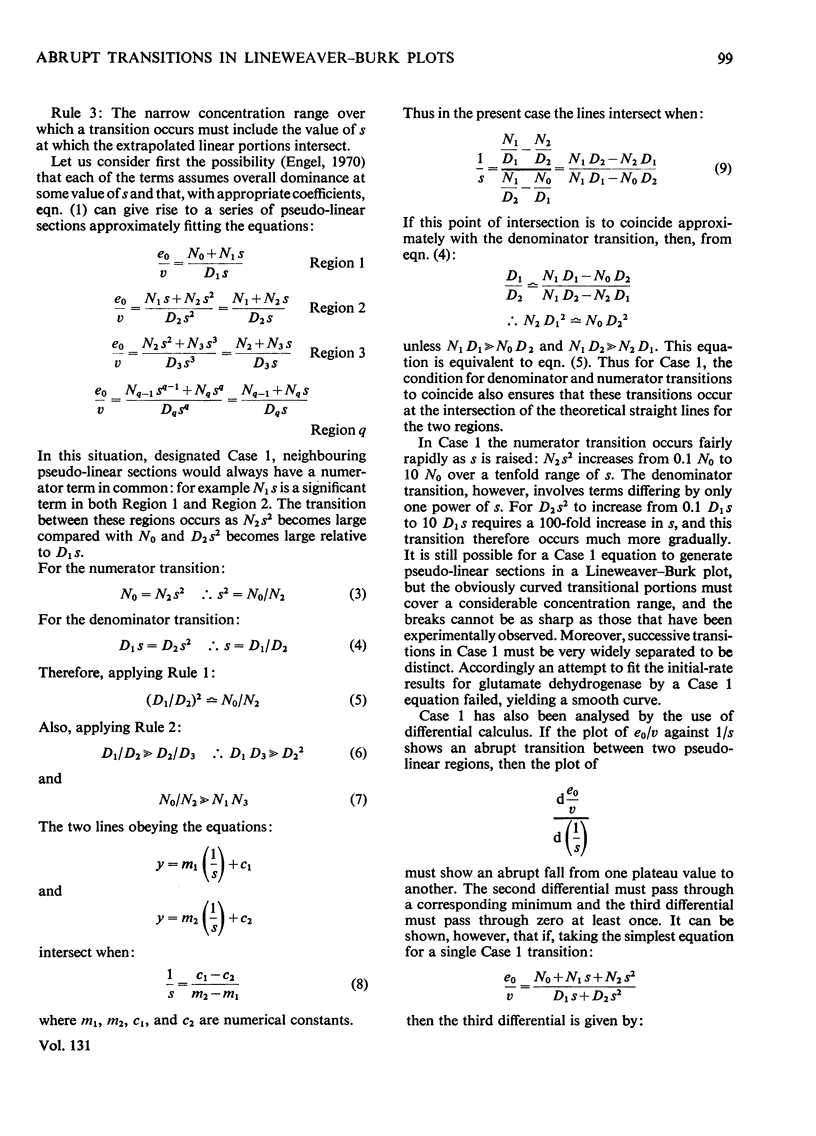
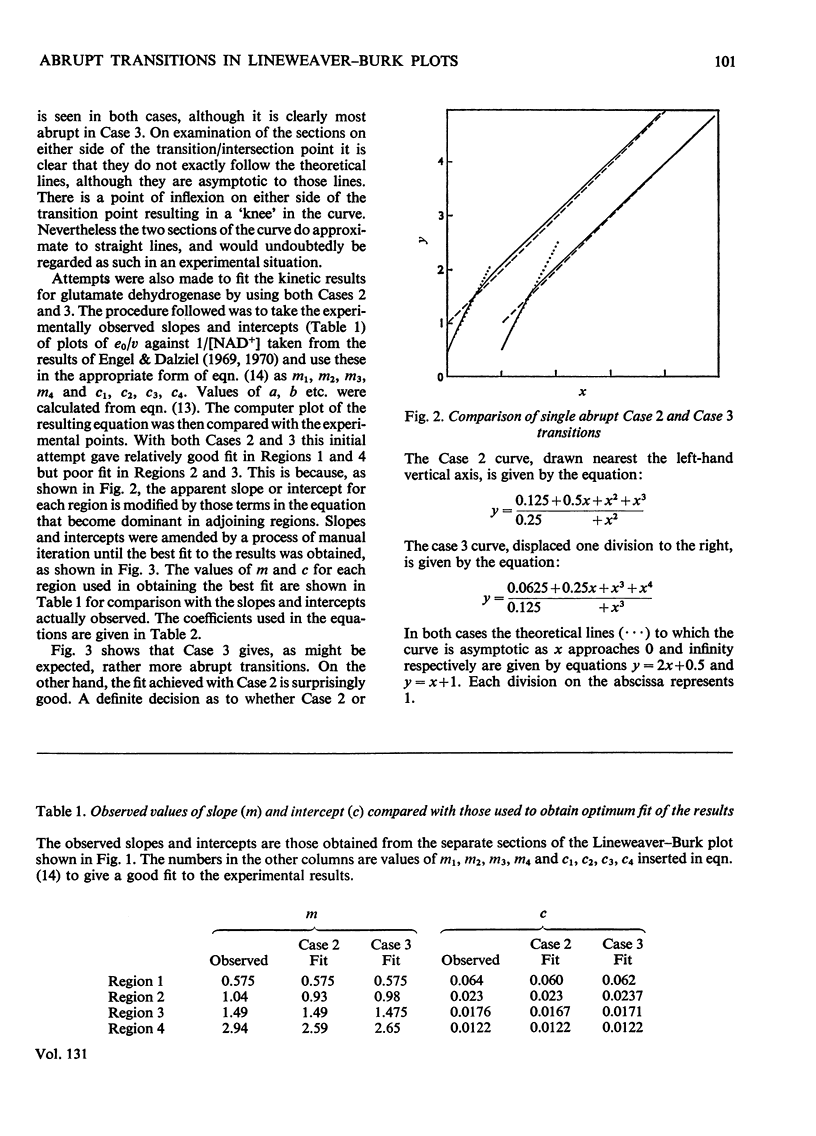
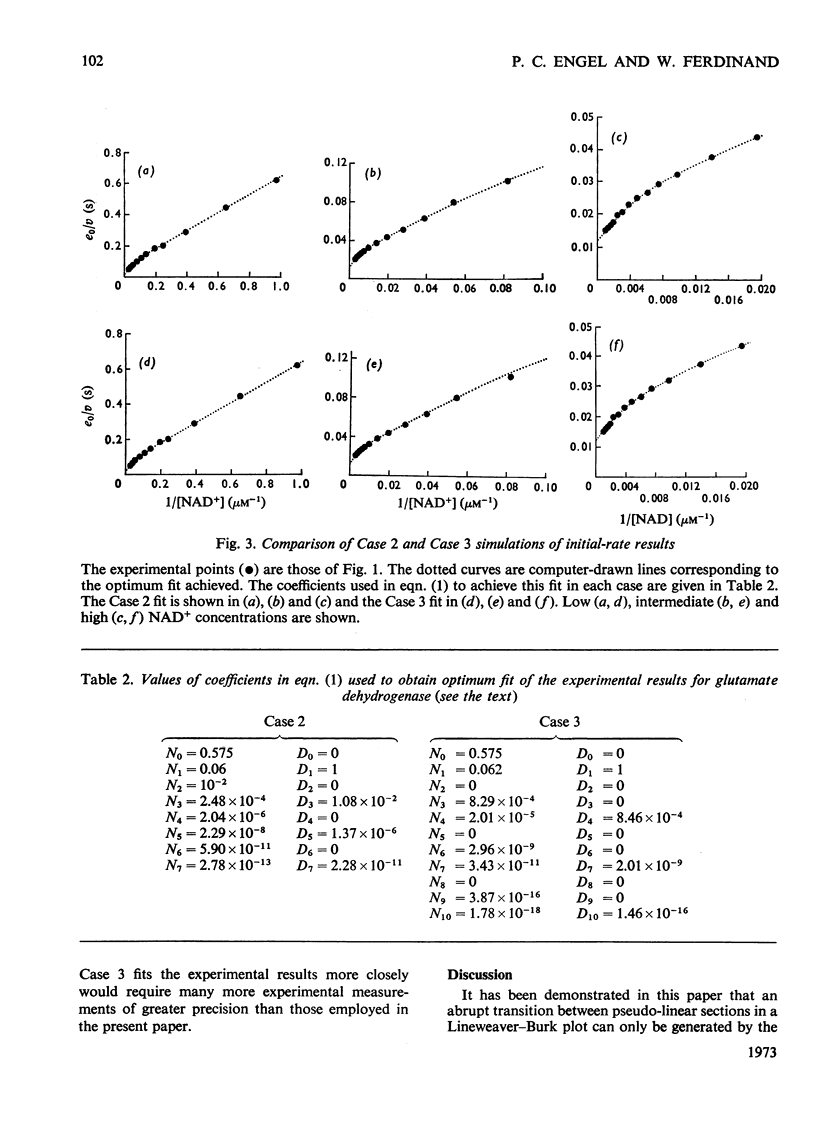
Abstract
1. Lineweaver–Burk plots for glutamate dehydrogenase, glucose 6-phosphate dehydrogenase and several other enzymes show one or more abrupt transitions between apparently linear sections. These transitions correspond to abrupt increases in the apparent Km and Vmax. with increasing concentration of the varied substrate. 2. The generalized reciprocal initial-rate equation for a multi-site enzyme requires several restrictions to be put on it in order to generate such plots. These mathematical conditions are explored. 3. It is shown that the effective omission of a term in the denominator of the reciprocal initial-rate equation represents a minimal requirement for generation of abrupt transitions. This corresponds in physical terms to negative co-operativity followed by positive co-operativity affecting the catalytic rate constant for the reaction. 4. Previous models for glutamate dehydrogenase cannot adequately account for the results. On the other hand, the model based on both negative and positive co-operativity gives a good fit to the experimental points. 5. The conclusions are discussed in relation to current knowledge of the structure and mechanism of glutamate dehydrogenase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. B., Horne R. N., Nordlie R. C. Glucose dehydrogenase activity of yeast glucose 6-phosphate dehydrogenase. II. Kinetic studies of the mode of activation by bicarbonate, phosphate, and sulfate. Biochemistry. 1968 Nov;7(11):3997–4004. doi: 10.1021/bi00851a029. [DOI] [PubMed] [Google Scholar]
- Barton J. S., Fisher J. R. Nonlinear kinetics of glutamate dehydrogenase. Studies with substrates--glutamate and nicotinamide-adenine dinucleotide. Biochemistry. 1971 Feb 16;10(4):577–585. doi: 10.1021/bi00780a006. [DOI] [PubMed] [Google Scholar]
- Cohen R., Mire M. Analytical-band centrifugation of an active enzyme-substrate complex. 2. Determination of active units of various enzymes. Eur J Biochem. 1971 Nov 11;23(2):276–281. doi: 10.1111/j.1432-1033.1971.tb01619.x. [DOI] [PubMed] [Google Scholar]
- Conway A., Koshland D. E., Jr Negative cooperativity in enzyme action. The binding of diphosphopyridine nucleotide to glyceraldehyde 3-phosphate dehydrogenase. Biochemistry. 1968 Nov;7(11):4011–4023. doi: 10.1021/bi00851a031. [DOI] [PubMed] [Google Scholar]
- Dalziel K., Egan R. R. The binding of oxidized coenzymes by glutamate dehydrogenase and the effects of glutarate and purine nucleotides. Biochem J. 1972 Feb;126(4):975–984. doi: 10.1042/bj1260975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel Keith, Engel Paul C. Antagonistic homotropic interactions as a possible explanation of coenzyme activation of glutamate dehydrogenase. FEBS Lett. 1968 Oct;1(5):349–352. doi: 10.1016/0014-5793(68)80153-x. [DOI] [PubMed] [Google Scholar]
- Di Prisco G. Tyrosyl and lysyl residues involved in the reactivity of catalytic and regulatory sites of crystalline beef liver glutamate dehydrogenase. Biochemistry. 1971 Feb 16;10(4):585–589. doi: 10.1021/bi00780a007. [DOI] [PubMed] [Google Scholar]
- Engel P. C., Dalziel K. Kinetic studies of glutamate dehydrogenase with glutamate and norvaline as substrates. Coenzyme activation and negative homotropic interactions in allosteric enzymes. Biochem J. 1969 Dec;115(4):621–631. doi: 10.1042/bj1150621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDEN C. Glutamic dehydrogenase. I. The effect of coenzyme on the sedimentation velocity and kinetic behavior. J Biol Chem. 1959 Apr;234(4):809–814. [PubMed] [Google Scholar]
- Fenn R. H., Marshall G. E. The stereochemical structure of disodium DL-glycerol 3-phosphate hexahydrate, the D isomer of which is an inhibitor of triose phosphate isomerase. Biochem J. 1972 Nov;130(1):1–10. doi: 10.1042/bj1300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourcade A., Venard R. Glutamate déshydrogénase de levure spécifique du NADP + : role du phosphate sur la réaction de désamination oxydative du glutamate. Biochim Biophys Acta. 1971 Aug 20;242(2):331–344. doi: 10.1016/0005-2744(71)90225-7. [DOI] [PubMed] [Google Scholar]
- Godinot C., Gautheron D. Regulation of pig heart mitochondrial glutamate dehydrogenase by nucleotides and phosphate: Comparison with pig heart and beef liver purified enzymes. FEBS Lett. 1971 Mar 16;13(4):235–240. doi: 10.1016/0014-5793(71)80543-4. [DOI] [PubMed] [Google Scholar]
- Jallon J. M., Iwatsubo M. Evidence for two nicotinamide binding sites on L-glutamate dehydrogenase. Biochem Biophys Res Commun. 1971 Nov;45(4):964–971. doi: 10.1016/0006-291x(71)90431-1. [DOI] [PubMed] [Google Scholar]
- Koberstein R., Sund H. Circular dichroism studies on the complex between beef liver glutamate dehydrogenase and NADH. FEBS Lett. 1971 Dec 1;19(2):149–151. doi: 10.1016/0014-5793(71)80500-8. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- LéJohn H. B., Jackson S. Allosteric interactions of a regulatory nicotinamide adenine dinucleotide-specific glutamate dehydrogenase from Blastocladiella. A molecular model for the enzyme. J Biol Chem. 1968 Jun 25;243(12):3447–3457. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Pinto P. V., Newton W. A., Jr, Richardson K. E. Evidence for four types of erythrocyte glucose-6-phosphate dehydrogenase from G-6-PD-deficient human subjects. J Clin Invest. 1966 Jun;45(6):823–831. doi: 10.1172/JCI105398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer J. A., Chiancone E., Yielding K. L., Antonini E. Intermediates in the reaction catalyzed by glutamate dehydrogenase. Eur J Biochem. 1972 Aug 4;28(4):528–532. doi: 10.1111/j.1432-1033.1972.tb01940.x. [DOI] [PubMed] [Google Scholar]
- Teipel J., Koshland D. E., Jr The significance of intermediary plateau regions in enzyme saturation curves. Biochemistry. 1969 Nov;8(11):4656–4663. doi: 10.1021/bi00839a064. [DOI] [PubMed] [Google Scholar]
- de Vijlder J. J., Slater E. C. The reaction between NAD+ and rabbit-muscle glyceraldehydephosphate dehydrogenase. Biochim Biophys Acta. 1968 Aug 27;167(1):23–34. doi: 10.1016/0005-2744(68)90274-x. [DOI] [PubMed] [Google Scholar]


