Abstract
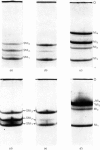
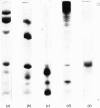
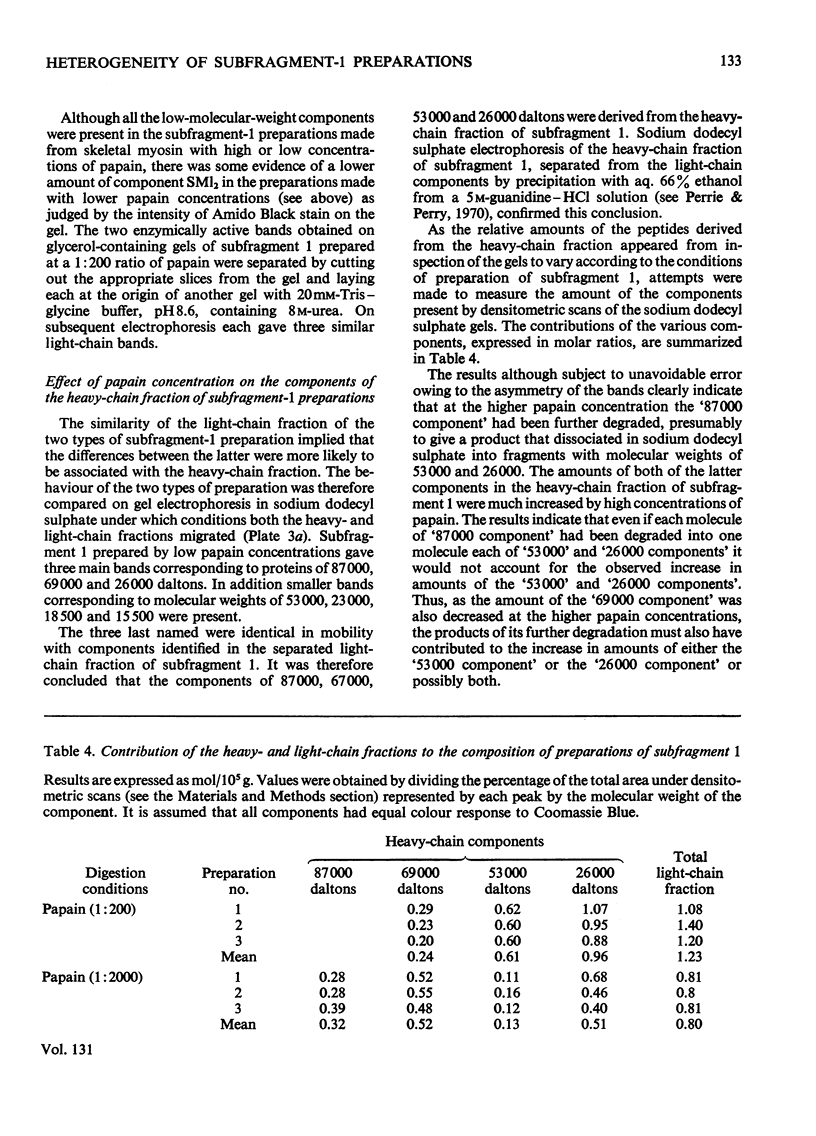
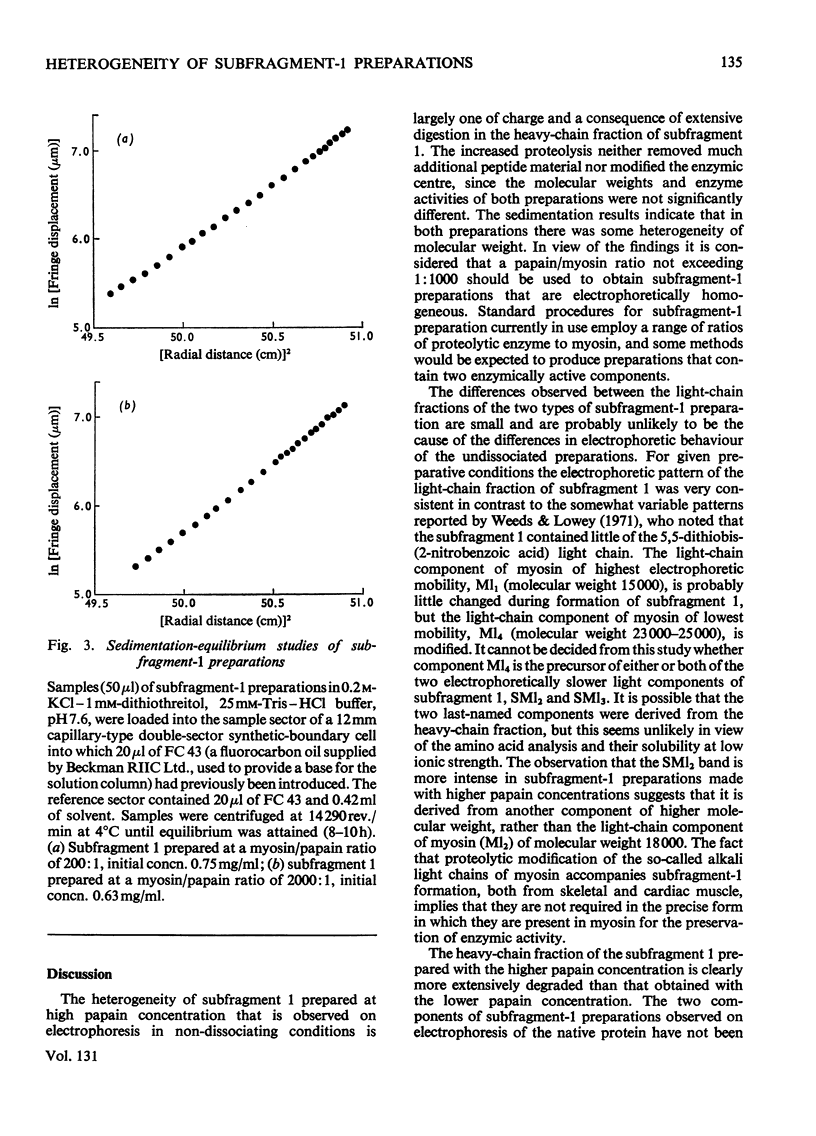
1. The physical, chemical and enzymic properties of subfragment 1 prepared from myosin of rabbit skeletal muscle by using two different concentrations of insoluble papain were compared. 2. Subfragment 1 prepared by using a myosin/papain ratio of 2000: 1 (by wt.) migrated on electrophoresis in non-dissociating conditions as a single enzymically active band. When prepared with a myosin/papain ratio of 200: 1 the preparation consisted of two enzymically active components of slightly different electrophoretic mobility. 3. The two types of preparation were obtained in similar yield and possessed similar specific adenosine triphosphatase activities when determined in the presence of Ca2+. 4. Gel electrophoresis in the presence of 8m-urea showed that both preparations contained three light components. The component of molecular weight 15500 was apparently identical with one of the light-chain components of myosin (Ml1). The other two light-chain components of subfragment 1 were not identical with any of the light-chain components of myosin. 5. The heavy-chain fraction of subfragment 1 prepared by using low concentrations of papain dissociated into components with molecular weights of 87000, 69000 and 26000 on electrophoresis in sodium dodecyl sulphate. The heavy-chain fraction of subfragment 1 prepared by using higher concentrations of papain contained components with molecular weights of 69000 and 53000 and relatively increased amounts of the component of molecular weight 26000. 6. The isolated 26000 dalton component had an amino acid composition similar to that of the heavy-chain fraction of subfragment 1 and contained 3-methylhistidine and mono-and tri-Nε-methyl-lysine. It was homogeneous on electrophoresis in the presence of sodium dodecyl sulphate but gave two bands on electrophoresis in 8m-urea.
Full text
PDF



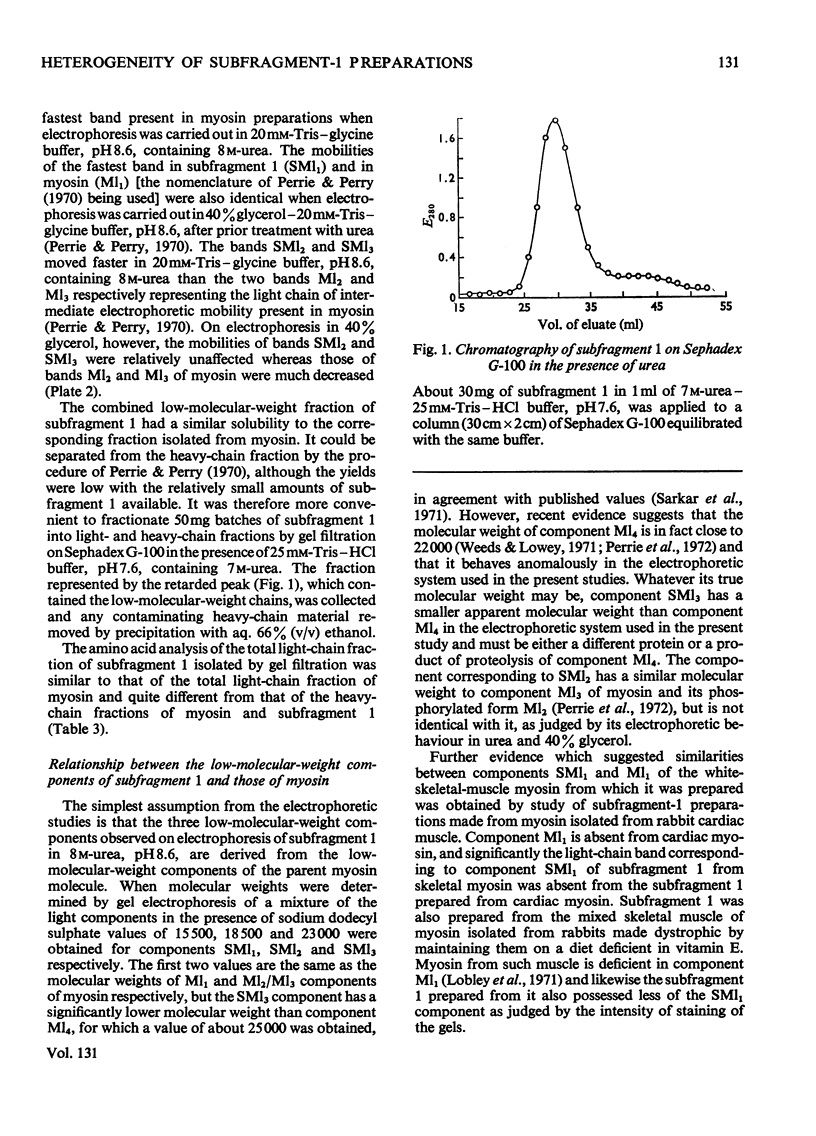


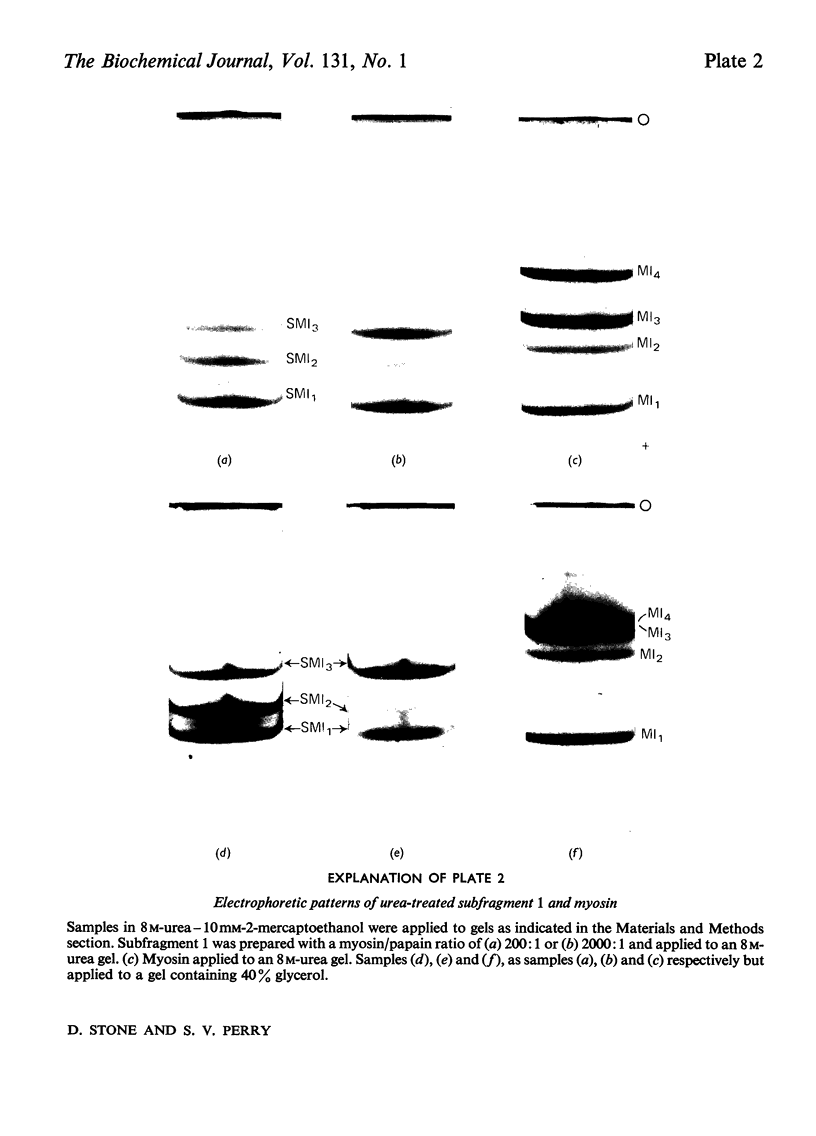




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akroyd P. Acrylamide gel slab electrophoresis in a simple glass cell for improved resolution and comparison of serum proteins. Anal Biochem. 1967 Jun;19(3):399–410. doi: 10.1016/0003-2697(67)90229-1. [DOI] [PubMed] [Google Scholar]
- Chervenka C. H. Long-column meniscus depletion sedimentation equilibrium technique for the analytical ultracentrifuge. Anal Biochem. 1970 Mar;34:24–29. doi: 10.1016/0003-2697(70)90082-5. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Hardy M. F., Harris C. I., Perry S. V., Stone D. Occurrence and formation of the N epsilon-methyl-lysines in myosin and the myofibrillar proteins. Biochem J. 1970 Dec;120(3):653–660. doi: 10.1042/bj1200653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. M., Perry S. V. The biological activity of subfragment 1 prepared from heavy meromyosin. Biochem J. 1966 Jul;100(1):120–129. doi: 10.1042/bj1000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobley G. E., Perry S. V., Stone D. Structural changes in myosin induced by vitamin E dystrophy. Nature. 1971 Jun 4;231(5301):317–318. doi: 10.1038/231317a0. [DOI] [PubMed] [Google Scholar]
- Lowey S., Slayter H. S., Weeds A. G., Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969 May 28;42(1):1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- MUELLER H., PERRY S. V. The chromatography of the meromyosins on diethylaminoethylcellulose. Biochem J. 1961 Jul;80:217–223. doi: 10.1042/bj0800217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUELLER H., PERRY S. V. The degradation of heavy meromyosin by trypsin. Biochem J. 1962 Dec;85:431–439. doi: 10.1042/bj0850431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller H. Characterization of the molecular region containing the active sites of myosin. J Biol Chem. 1965 Oct;240(10):3816–3828. [PubMed] [Google Scholar]
- Nauss K. M., Kitagawa S., Gergely J. Pyrophosphate binding to and adenosine triphosphatase activity of myosin and its proteolytic fragments. Implications for the substructure of myosin. J Biol Chem. 1969 Feb 25;244(4):755–765. [PubMed] [Google Scholar]
- Perrie W. T., Perry S. V. An electrophoretic study of the low-molecular-weight components of myosin. Biochem J. 1970 Aug;119(1):31–38. doi: 10.1042/bj1190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Sreter F. A., Gergely J. Light chains of myosins from white, red, and cardiac muscles. Proc Natl Acad Sci U S A. 1971 May;68(5):946–950. doi: 10.1073/pnas.68.5.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayter H. S., Lowey S. Substructure of the myosin molecule as visualized by electron microscopy. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1611–1618. doi: 10.1073/pnas.58.4.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WACHSTEIN M., MEISEL E. Histochemistry of hepatic phosphatases of a physiologic pH; with special reference to the demonstration of bile canaliculi. Am J Clin Pathol. 1957 Jan;27(1):13–23. doi: 10.1093/ajcp/27.1.13. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weeds A. G., Lowey S. Substructure of the myosin molecule. II. The light chains of myosin. J Mol Biol. 1971 Nov 14;61(3):701–725. doi: 10.1016/0022-2836(71)90074-x. [DOI] [PubMed] [Google Scholar]
- YOUNG D. M., HIMMELFARB S., HARRINGTON W. F. ON THE STRUCTURAL ASSEMBLY OF THE POLYPEPTIDE CHAINS OF HEAVY MEROMYOSIN. J Biol Chem. 1965 Jun;240:2428–2436. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]