Abstract
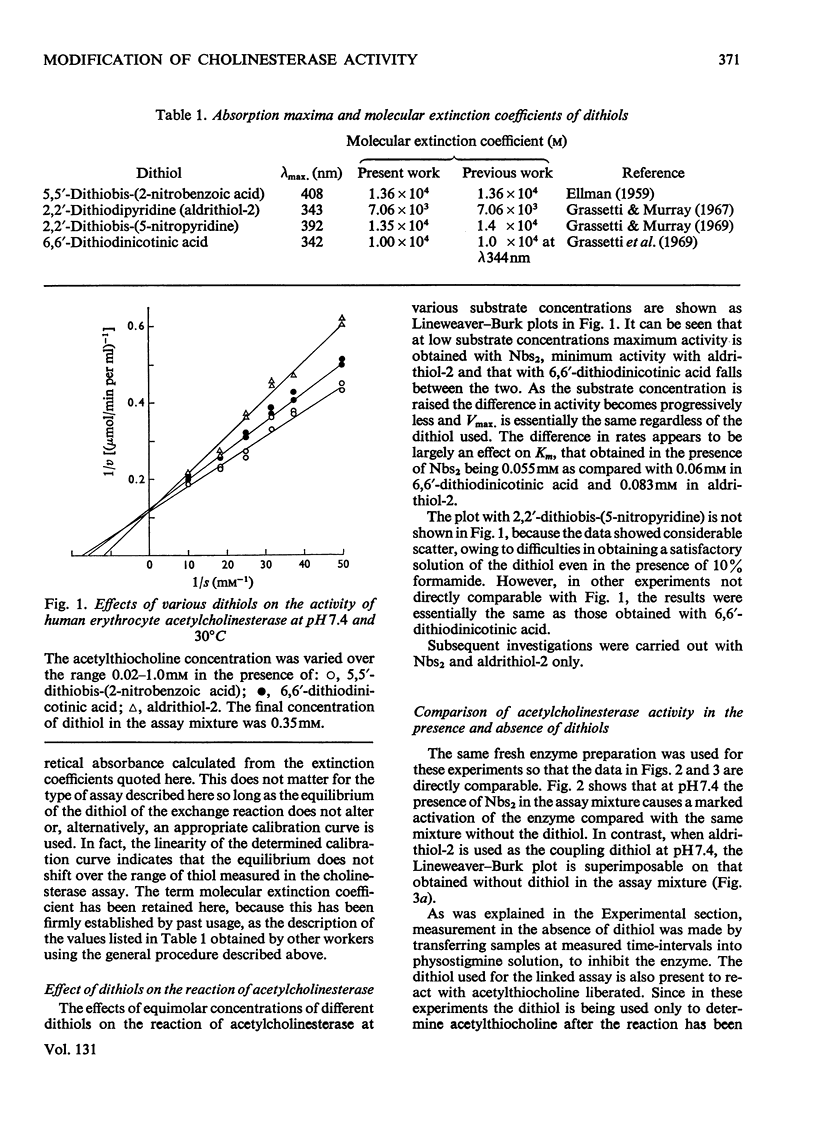
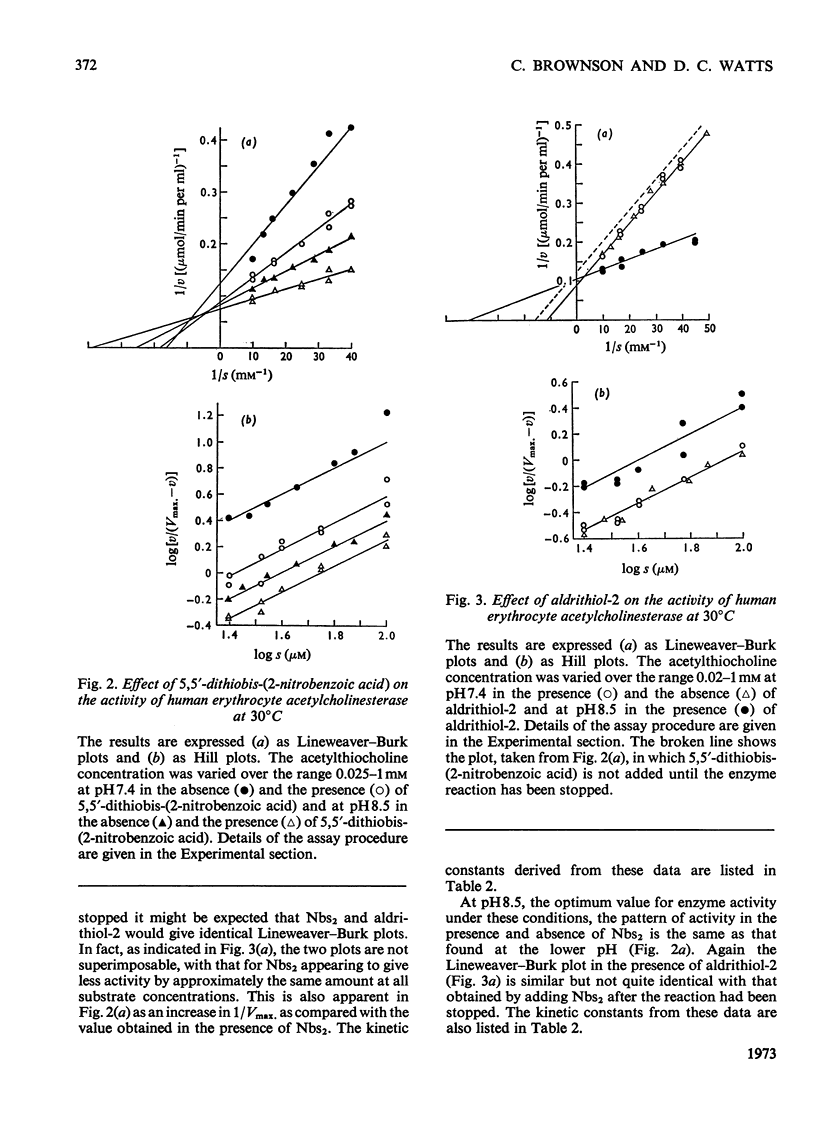
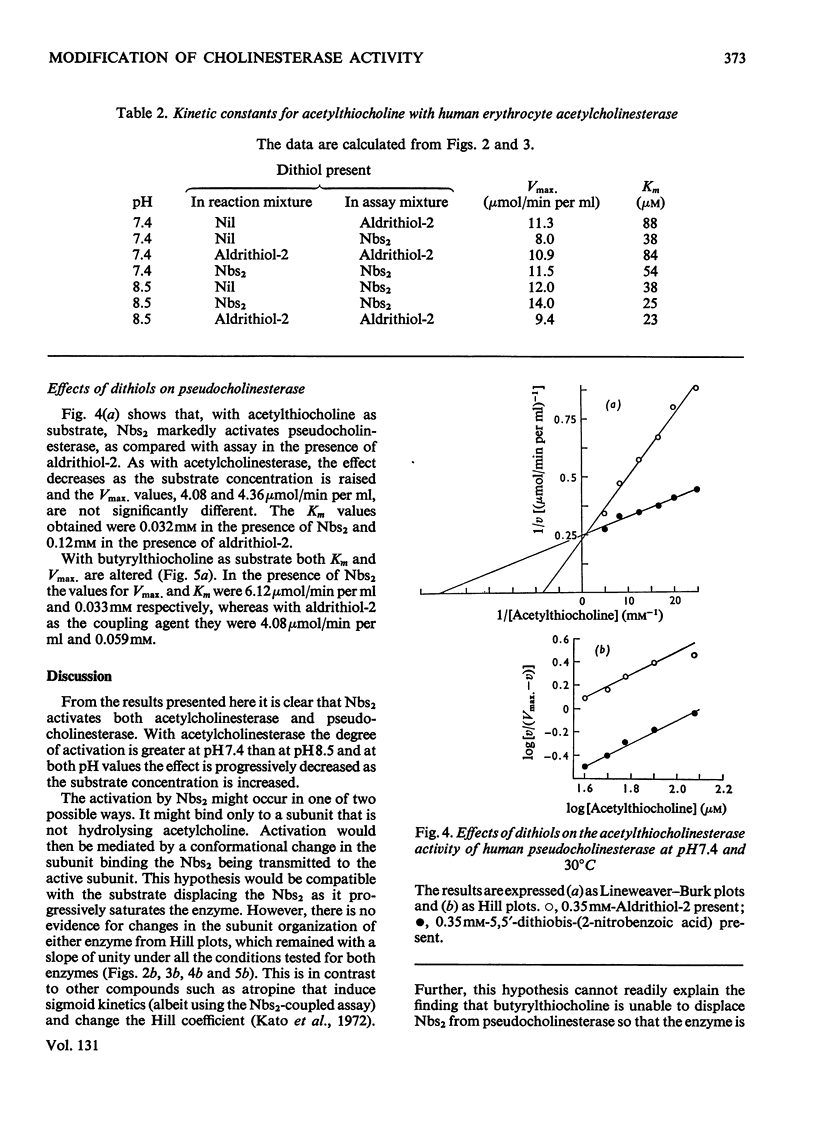
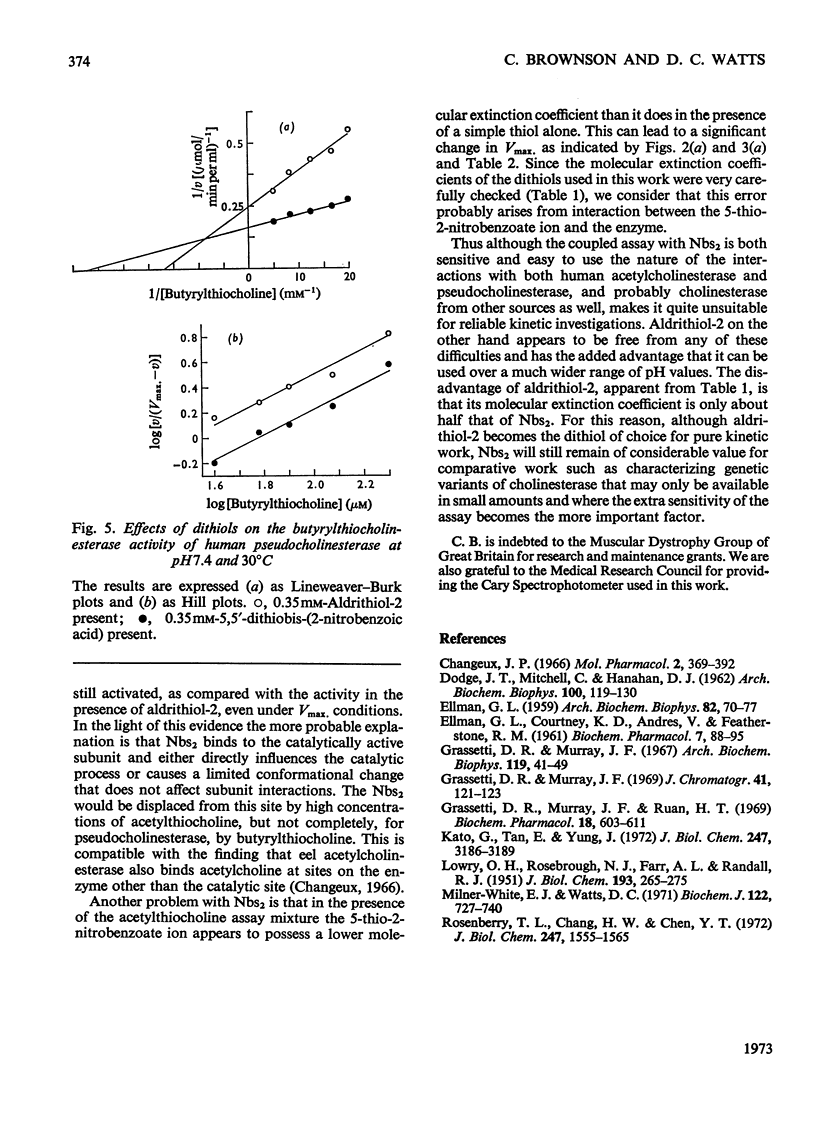
1. Compared with the acetylcholinesterase assay carried out in the absence of a dithiol, the presence of 5,5′-dithiobis-(2-nitrobenzoic acid) caused marked activation, 6,6′-dithiodinicotinic acid and 2,2′-dithiobis-(5-nitropyridine) less so and 2,2′-dithiodipyridine (aldrithiol-2) had no effect at all. Measurements are further complicated in that the 5-thio-2-nitrobenzoate ion also appears to interact with the enzyme, resulting in slightly lowered absorbance values. 2. Acetylthiocholine competes for the 5,5′-dithiobis-(2-nitrobenzoic acid)-binding site so that activation is essentially eliminated by saturating concentrations of substrate. The presence of the dithiol decreases the Km value of acetylthiocholine. 3. Similar results were obtained with pseudocholinesterase. However, with butyrylthiocholine clear activation was still observed under Vmax. conditions in addition to Km being lowered. 4. All the data yielded Hill coefficients of 1 and analysis of the results leads to the conclusion that activation results from the dithiol being bound to a site on the subunit that is actively catalysing ester hydrolysis. 5. The use of aldrithiol-2 is recommended for kinetic work where absolute quantitative measurements are required.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Changeux J. P. Responses of acetylcholinesterase from Torpedo marmorata to salts and curarizing drugs. Mol Pharmacol. 1966 Sep;2(5):369–392. [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Grassetti D. R., Murray J. F., Jr Determination of sulfhydryl groups with 2,2'- or 4,4'-dithiodipyridine. Arch Biochem Biophys. 1967 Mar;119(1):41–49. doi: 10.1016/0003-9861(67)90426-2. [DOI] [PubMed] [Google Scholar]
- Grassetti D. R., Murray J. F., Jr, Ruan H. T. The interaction of 6,6'-dithiodinicotinic acid with thiols and with ehrlich ascites tumor cells. Biochem Pharmacol. 1969 Mar;18(3):603–611. doi: 10.1016/0006-2952(69)90085-9. [DOI] [PubMed] [Google Scholar]
- Grassetti D. R., Murray J. F., Jr The use of 2,2'-dithiobis-(5-nitropyridine) as a selective reagent for the detection of thiols. J Chromatogr. 1969 Apr 22;41(1):121–123. doi: 10.1016/0021-9673(64)80109-6. [DOI] [PubMed] [Google Scholar]
- Kato G., Tan E., Yung J. Acetylcholinesterase. Kinetic studies on the mechanism of atropine inhibition. J Biol Chem. 1972 May 25;247(10):3186–3189. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Milner-White E. J., Watts D. C. Inhibition of adenosine 5'-triphosphate-creatine phosphotransferase by substrate-anion complexes. Evidence for the transition-state organization of the catalytic site. Biochem J. 1971 May;122(5):727–740. doi: 10.1042/bj1220727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberry T. L., Chang H. W., Chen Y. T. Purification of acetylcholinesterase by affinity chromatography and determination of active site stoichiometry. J Biol Chem. 1972 Mar 10;247(5):1555–1565. [PubMed] [Google Scholar]


