Abstract
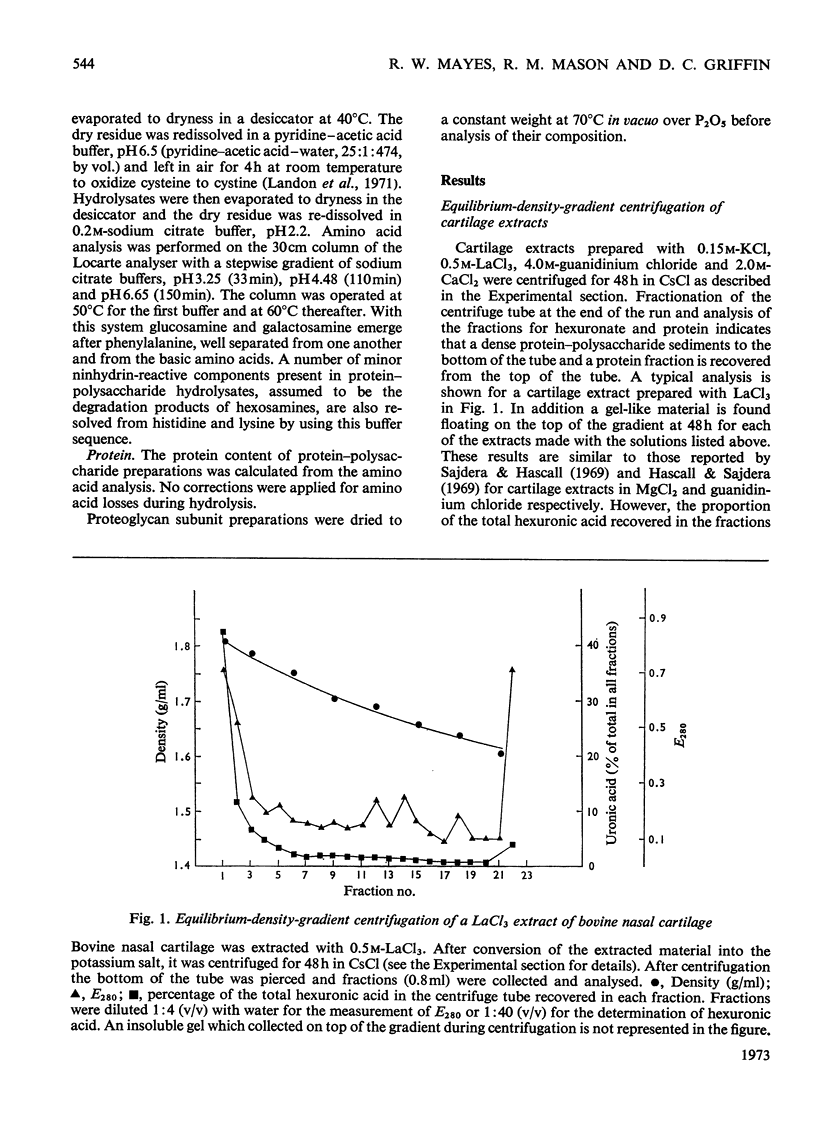
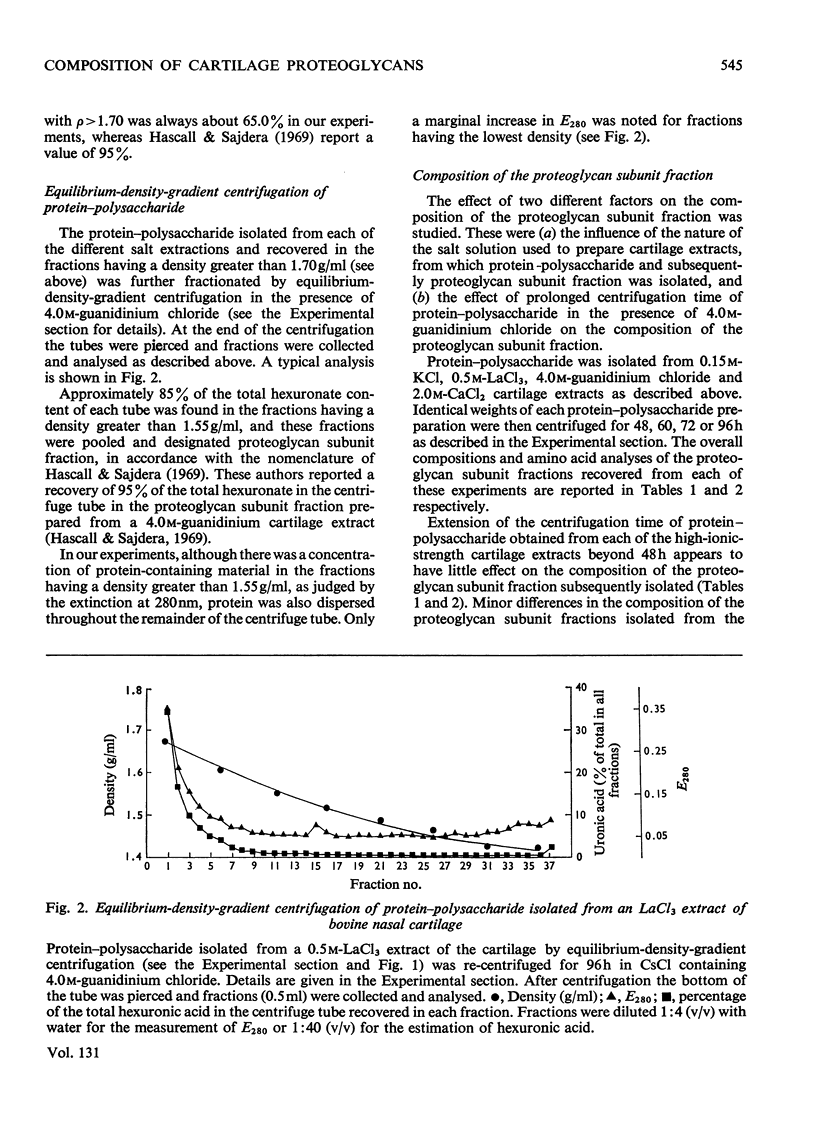
1. A proteoglycan fraction (the proteoglycan subunit fraction) was prepared from extracts, with 0.15m-KCl (low-ionic-strength) and 0.5m-LaCl3, 2.0m-CaCl2 and 4.0m-guanidinium chloride (high-ionic-strength), of bovine nasal cartilage by equilibrium-density-gradient centrifugation, essentially as described by Hascall & Sajdera (1969). 2. The use of different centrifugation times showed that near-equilibrium conditions were reached by 48h for the fractions prepared from the high-ionic-strength extracts. The fraction isolated from the low-ionic-strength extract required a longer centrifugation time to reach equilibrium conditions. 3. The composition of the proteoglycan fractions from the various extracts was compared by analyses of their carbohydrate and amino acid contents. Difference indices were calculated from the amino acid analysis to compare the degree of compositional relationship between the protein components of the proteoglycans. 4. Small compositional differences were found between the proteoglycans isolated from the various high-ionic-strength extracts. The protein content of the fractions from the CaCl2 extract and the guanidinium chloride extract showed the greatest difference in this respect, although their amino acid analysis was similar. 5. The proteoglycan fraction isolated from the low-ionic-strength extract shows marked differences in composition from the fractions isolated from the high-ionic-strength extracts. Its protein and glucosamine contents were lower whereas its hexuronic acid and galactosamine contents were higher than those of the latter. It also exhibits major differences in its amino acid composition. The glucosamine:galactosamine ratio of the fraction from the low-ionic-strength extract indicates that it may be an almost exclusively chondroitin sulphate–proteoglycan. Its analysis correlates closely with that of a low-molecular-weight proteoglycan isolated from pig laryngeal cartilage by Tsiganos & Muir (1969). 6. The proteoglycan fractions from both the low- and high-ionic-strength extracts migrate as a single band in zone electrophoresis carried out in a sucrose-density gradient at both pH3.0 and pH7.0, although each showed evidence of band widening during the electrophoresis. All the proteoglycan fractions migrated with the same electrophoretic mobility at pH3.0, irrespective of the differences in composition between them. 7. The differences between the proteoglycans from the low- and high-ionic-strength extracts are discussed and the view is advanced that they may be due to association between predominantly chondroitin sulphate–proteoglycans and a keratan sulphate-enriched proteoglycan species.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Brakke M. K., Allington R. W., Langille F. A. Mobility measurements by photometric analysis of zone electrophoresis in a sucrose gradient column. Anal Biochem. 1968 Oct 24;25(1):30–39. doi: 10.1016/0003-2697(68)90077-8. [DOI] [PubMed] [Google Scholar]
- DOGANGES P. T., SCHUBERT M. THE USE OF LANTHANUM TO STUDY THE DEGRADATION OF A PROTEINPOLYSACCHARIDE FROM CARTILAGE. J Biol Chem. 1964 May;239:1498–1503. [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Physical properties and polydispersity of proteoglycan from bovine nasal cartilage. J Biol Chem. 1970 Oct 10;245(19):4920–4930. [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Landon M., Melamed M. D., Smith E. L. Sequence of bovine liver glutamate dehydrogenase. I. Isolation of tryptic peptides from the carboxymethylated protein. J Biol Chem. 1971 Apr 25;246(8):2360–2373. [PubMed] [Google Scholar]
- Mashburn T. A., Jr, Hoffman P. Comparative fractionation studies of cartilage proteinpolysaccharides. J Biol Chem. 1971 Nov;246(21):6497–6506. [PubMed] [Google Scholar]
- Mason R. M., Mayes R. W. Extraction of cartilage protein-polysaccharides with inorganic salt solutions. Biochem J. 1973 Mar;131(3):535–540. doi: 10.1042/bj1310535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. M. Observations on the glycosaminoglycans of aging bronchial cartilage studied with Alcian Blue. Histochem J. 1971 Nov;3(6):421–434. doi: 10.1007/BF01014780. [DOI] [PubMed] [Google Scholar]
- Mason R. M. Protein-polysaccharide complexes from adult human tracheal cartilage. Biochem J. 1970 Sep;119(3):599–601. doi: 10.1042/bj1190599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. M., Wusteman F. S. The glycosaminoglycans of human tracheobronchial cartilage. Biochem J. 1970 Dec;120(4):777–785. doi: 10.1042/bj1200777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger H., Shapiro M. B., Mosimann J. E., Vinton J. E. Assessment of compositional relatedness between proteins. Nature. 1968 Sep 14;219(5159):1166–1168. doi: 10.1038/2191166a0. [DOI] [PubMed] [Google Scholar]
- Pal S., Doganges P. T., Schubert M. The separation of new forms of the proteinpolysaccharides of bovine nasal cartilage. J Biol Chem. 1966 Sep 25;241(18):4261–4266. [PubMed] [Google Scholar]
- Rodén L., Smith R. Structure of the neutral trisaccharide of the chondroitin 4-sulfate-protein linkage region. J Biol Chem. 1966 Dec 25;241(24):5949–5954. [PubMed] [Google Scholar]
- Rosenberg L., Hellmann W., Kleinschmidt A. K. Macromolecular models of proteinpolysaccharides from bovine nasal cartilage based on electron microscopic studies. J Biol Chem. 1970 Aug 25;245(16):4123–4130. [PubMed] [Google Scholar]
- Rosenberg L., Pal S., Beale R., Schubert M. A comparison of proteinpolysaccharides of bovine nasal cartilage isolated and fractionated by different methods. J Biol Chem. 1970 Aug 25;245(16):4112–4122. [PubMed] [Google Scholar]
- Sajdera S. W., Hascall V. C. Proteinpolysaccharide complex from bovine nasal cartilage. A comparison of low and high shear extraction procedures. J Biol Chem. 1969 Jan 10;244(1):77–87. [PubMed] [Google Scholar]
- Schubert M. Structure of connective tissues, a chemical point of view. Fed Proc. 1966 May-Jun;25(3):1047–1052. [PubMed] [Google Scholar]
- Simpson D. L., Davidson E. A. Isolation of the peptide core of costal cartilage chondroitin 4-sulfate proteoglycan. Biochemistry. 1972 May 9;11(10):1856–1861. doi: 10.1021/bi00760a020. [DOI] [PubMed] [Google Scholar]
- Tsiganos C. P., Muir H. Studies on protein-polysaccharides from pig laryngeal cartilage. Extraction and purification. Biochem J. 1969 Aug;113(5):879–884. doi: 10.1042/bj1130879. [DOI] [PMC free article] [PubMed] [Google Scholar]


