Abstract
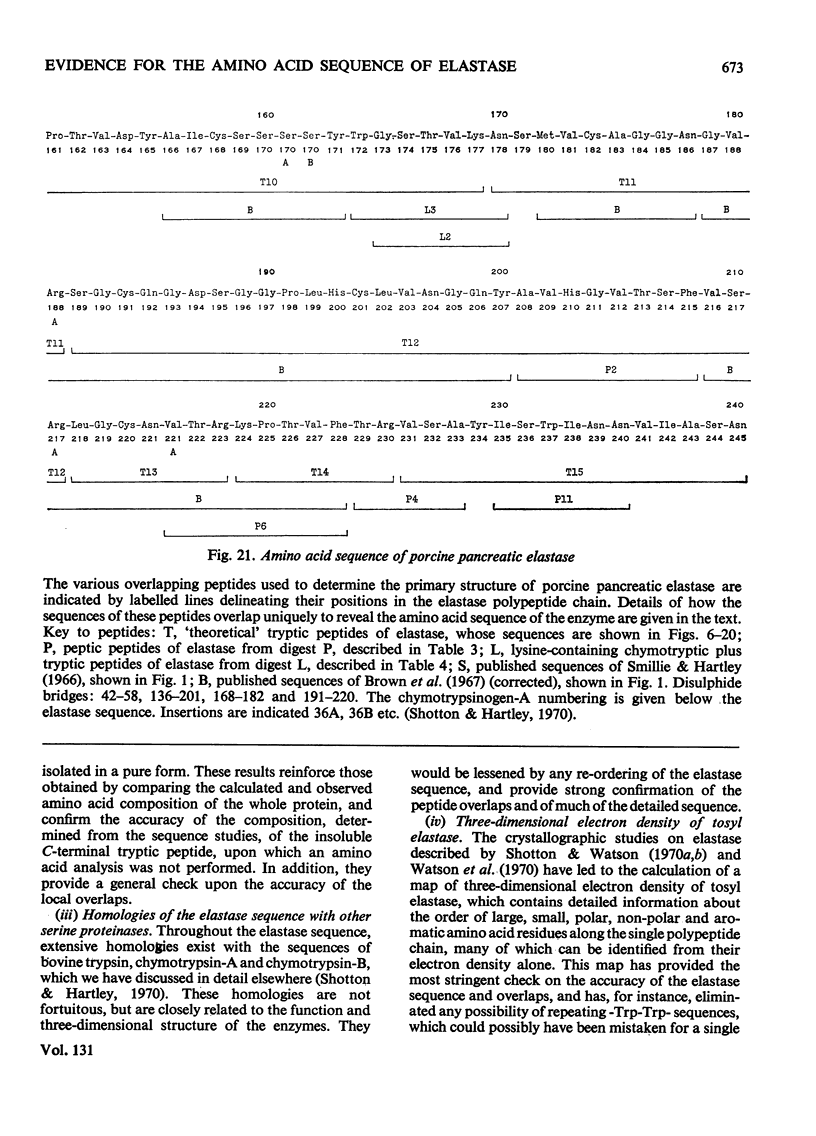
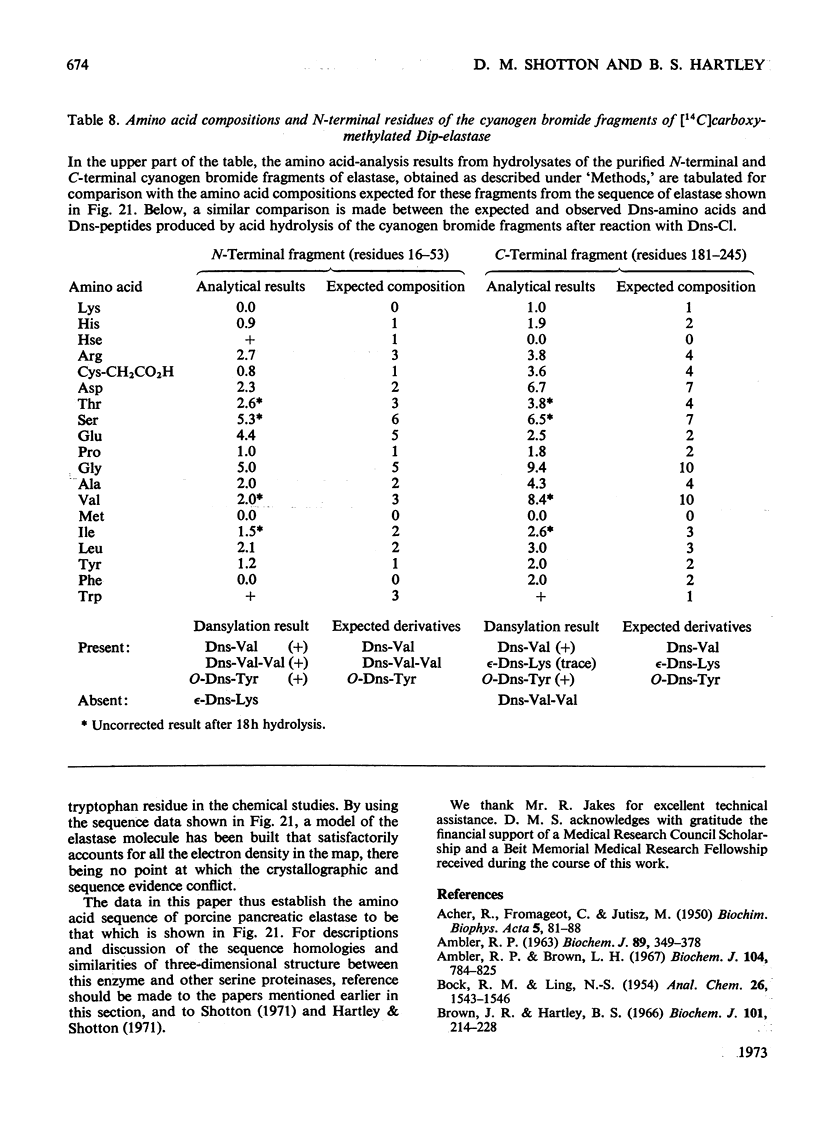
The preparation and purification of tryptic peptides from aminoethylated Dip-elastase and [14C]carboxymethylated Dip-elastase, and of peptic peptides from native elastase is described. A summary of the results of chemical studies used to elucidate the amino acid sequence of these peptides is presented. Full details are given in a supplementary paper that has been deposited as Supplementary Publication SUP 50016 at the National Lending Library for Science and Technology, Boston Spa, Yorks. LS23 7BQ, U.K., from whom copies can be obtained on the terms indicated in Biochem. J. (1973), 131, 1–20. These results, together with those from previously published papers, are used to establish the complete amino acid sequence of elastase, which is a single polypeptide chain of 240 residues, molecular weight 25900, containing four disulphide bridges.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACHER R., FROMAGEOT C., JUTISZ M. Séparations chromatographiques d'acides aminés et de peptides; les acides aminés et de peptides; les acides aminés individuels de l'insuline avec une note sur le dosage de la proline. Biochim Biophys Acta. 1950 Mar;5(1):81–88. doi: 10.1016/0006-3002(50)90151-x. [DOI] [PubMed] [Google Scholar]
- AMBLER R. P. THE AMINO ACID SEQUENCE OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:349–378. doi: 10.1042/bj0890349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P., Brown L. H. The amino acid sequence of Pseudomonas fluorescens azurin. Biochem J. 1967 Sep;104(3):784–825. doi: 10.1042/bj1040784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARKER S. A., BOURNE E. J., STACEY M., WARD R. B. Some paper-chromatographic studies with Aspergillus niger '152' transfructosylase. Biochem J. 1958 May;69(1):60–62. doi: 10.1042/bj0690060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. R., Hartley B. S. Location of disulphide bridges by diagonal paper electrophoresis. The disulphide bridges of bovine chymotrypsinogen A. Biochem J. 1966 Oct;101(1):214–228. doi: 10.1042/bj1010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. R., Kauffman D. L., Hartley B. S. The primary structure of porcine pancreatic elastase. The N-terminus and disulphide bridges. Biochem J. 1967 May;103(2):497–507. doi: 10.1042/bj1030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPLIN H., COHEN S., PRESS E. M. PREPARATION AND PROPERTIES OF THE PEPTIDE CHAINS OF NORMAL HUMAN 19 S GAMMA-GLOBULIN (IGM). Biochem J. 1965 Apr;95:256–261. doi: 10.1042/bj0950256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALGLIESH C. E. The relation between pyridoxin and tryptophan metabolism, studied in the rat. Biochem J. 1952 Sep;52(1):3–14. doi: 10.1042/bj0520003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlanger B. F., Cooper A. G., Cohen W. The inactivation of chymotrypsin by diphenylcarbamyl chloride and its reactivation by nucleophilic agents. Biochemistry. 1966 Jan;5(1):190–196. doi: 10.1021/bi00865a025. [DOI] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., HARRIS J. I., LEVY A. L. Recent developments in techniques for terminal and sequence studies in peptides and proteins. Methods Biochem Anal. 1955;2:359–425. doi: 10.1002/9780470110188.ch12. [DOI] [PubMed] [Google Scholar]
- GRAY W. R., HARTLEY B. S. THE STRUCTURE OF A CHYMOTRYPTIC PEPTIDE FROM PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:379–380. doi: 10.1042/bj0890379. [DOI] [PubMed] [Google Scholar]
- GROSS E., WITKOP B. Nonenzymatic cleavage of peptide bonds: the methionine residues in bovine pancreatic ribonuclease. J Biol Chem. 1962 Jun;237:1856–1860. [PubMed] [Google Scholar]
- Gertler A., Hofmann T. The involvement of the amino-terminal amino acid in the activity of pancreatic proteases. I. The effects of nitrous acid on elastase. J Biol Chem. 1967 May 25;242(10):2522–2527. [PubMed] [Google Scholar]
- HARTLEY B. S., NAUGHTON M. A., SANGER F. The amino acid sequence around the reactive serine of elastase. Biochim Biophys Acta. 1959 Jul;34:243–244. doi: 10.1016/0006-3002(59)90254-9. [DOI] [PubMed] [Google Scholar]
- HARTLEY B. S. Proteolytic enzymes. Annu Rev Biochem. 1960;29:45–72. doi: 10.1146/annurev.bi.29.070160.000401. [DOI] [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irreverre F. A modified Sakaguchi spray. Biochim Biophys Acta. 1965 Dec 16;111(2):551–552. doi: 10.1016/0304-4165(65)90069-3. [DOI] [PubMed] [Google Scholar]
- JEPSON J. B., SMITH I. Multiple dipping procedures in paper chromatography: a specific test for hydroxy-proline. Nature. 1953 Dec 12;172(4389):1100–1101. doi: 10.1038/1721100b0. [DOI] [PubMed] [Google Scholar]
- Kaplan H., Stevenson K. J., Hartley B. S. Competitive labelling, a method for determining the reactivity of individual groups in proteins. The amino groups of porcine elastase. Biochem J. 1971 Sep;124(2):289–299. doi: 10.1042/bj1240289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C. Immunoglobulin kappa-chains. Comparative sequences in selected stretches of Bence-Jones proteins. Biochem J. 1966 Nov;101(2):352–368. doi: 10.1042/bj1010352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C. The disulphide bridges of immunoglobulin kappa-chains. Biochem J. 1966 Nov;101(2):338–351. doi: 10.1042/bj1010338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAUGHTON M. A., SANGER F., HARTLEY B. S., SHAW D. C. The amino acid sequence around the reactive serine residue of some proteolytic enzymes. Biochem J. 1960 Oct;77:149–163. doi: 10.1042/bj0770149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAUGHTON M. A., SANGER F. Purification and specificity of pancreatic elastase. Biochem J. 1961 Jan;78:156–163. doi: 10.1042/bj0780156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Partridge S. M. Filter-paper partition chromatography of sugars: 1. General description and application to the qualitative analysis of sugars in apple juice, egg white and foetal blood of sheep. with a note by R. G. Westall. Biochem J. 1948;42(2):238–250. doi: 10.1042/bj0420238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plapp B. V., Raftery M. A., Cole R. D. The tryptic digestion of S-aminoethylated ribonuclease. J Biol Chem. 1967 Jan 25;242(2):265–270. [PubMed] [Google Scholar]
- Raftery M. A., Cole R. D. On the aminoethylation of proteins. J Biol Chem. 1966 Aug 10;241(15):3457–3461. [PubMed] [Google Scholar]
- Shotton D. M., Hartley B. S. Amino-acid sequence of porcine pancreatic elastase and its homologies with other serine proteinases. Nature. 1970 Feb 28;225(5235):802–806. doi: 10.1038/225802a0. [DOI] [PubMed] [Google Scholar]
- Shotton D. M., Watson H. C. The three-dimensional structure of crystalline porcine pancreatic elastase. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):111–118. doi: 10.1098/rstb.1970.0013. [DOI] [PubMed] [Google Scholar]
- Shotton D. M., Watson H. C. Three-dimensional structure of tosyl-elastase. Nature. 1970 Feb 28;225(5235):811–816. doi: 10.1038/225811a0. [DOI] [PubMed] [Google Scholar]
- Smillie L. B., Hartley B. S. Histidine sequences in the active centres of some 'serine' proteinases. Biochem J. 1966 Oct;101(1):232–241. doi: 10.1042/bj1010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALEY S. G., WATSON J. The action of trypsin on polylysine. Biochem J. 1953 Sep;55(2):328–337. doi: 10.1042/bj0550328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson H. C., Shotton D. M., Cox J. M., Muirhead H. Three-dimensional Fourier synthesis of tosyl-elastase at 3.5 å resolution. Nature. 1970 Feb 28;225(5235):806–811. doi: 10.1038/225806a0. [DOI] [PubMed] [Google Scholar]


