Abstract
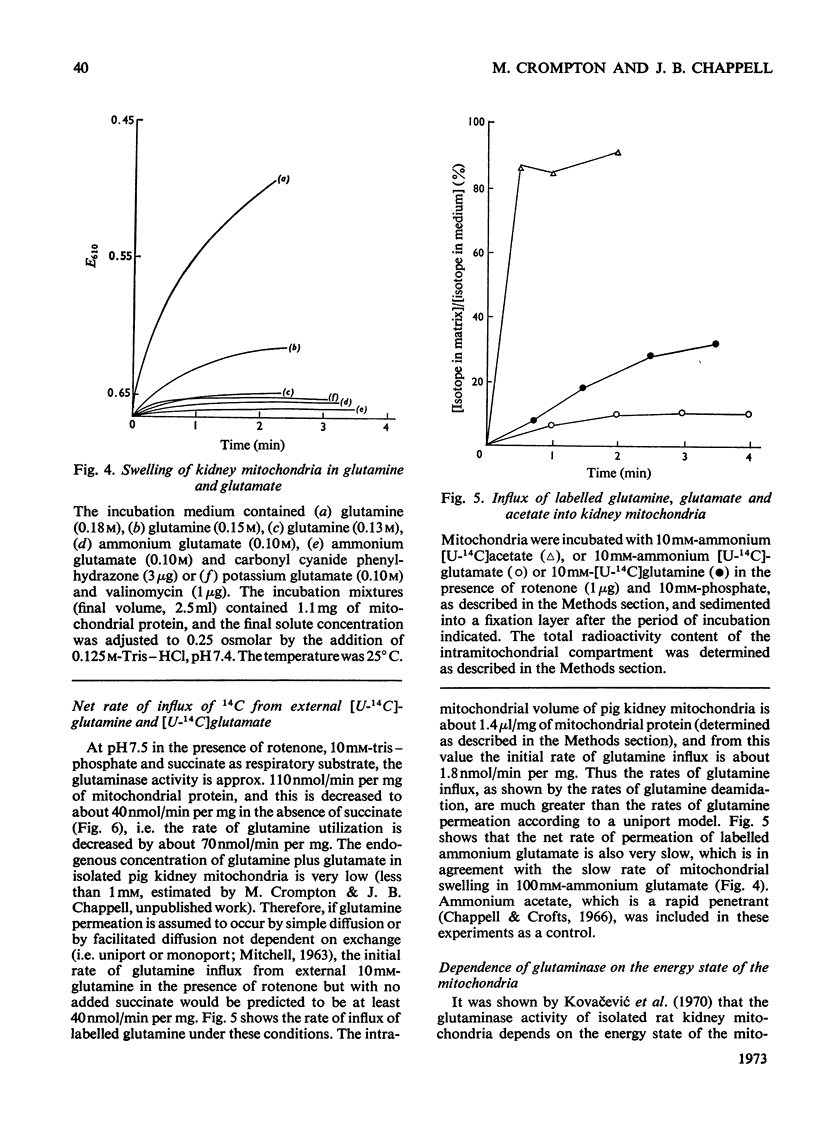
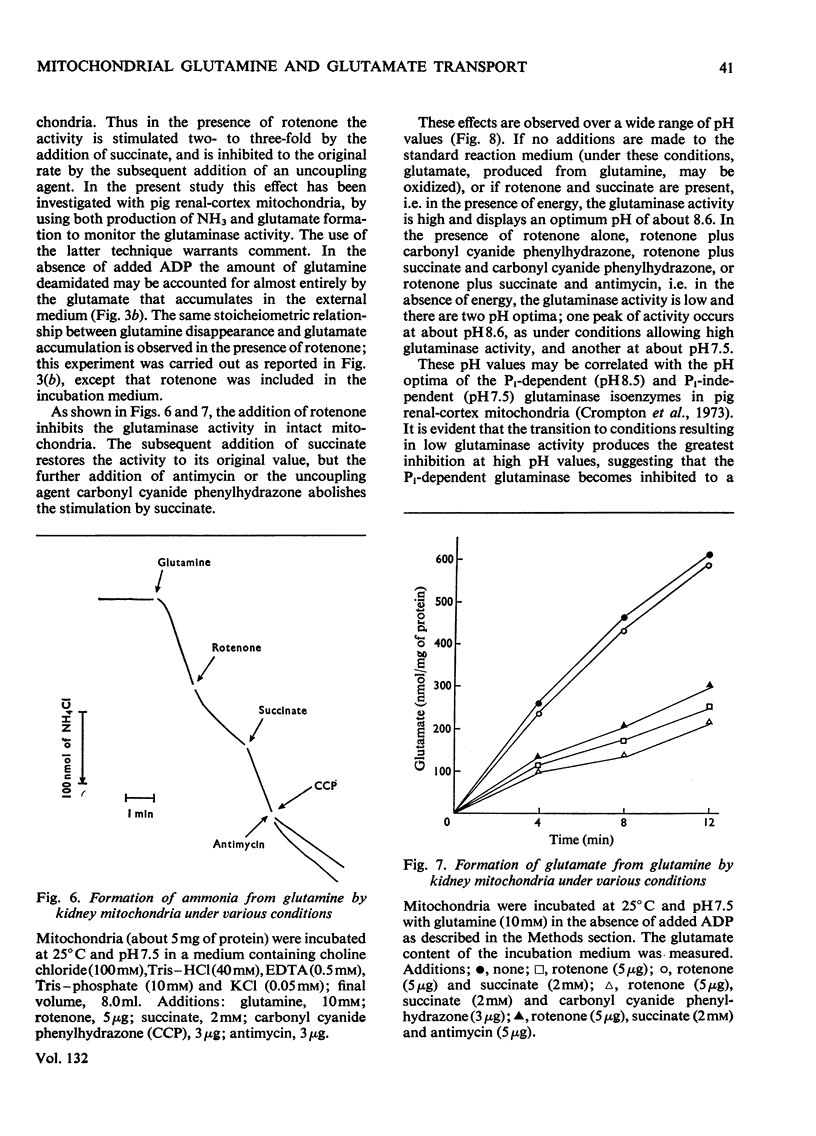
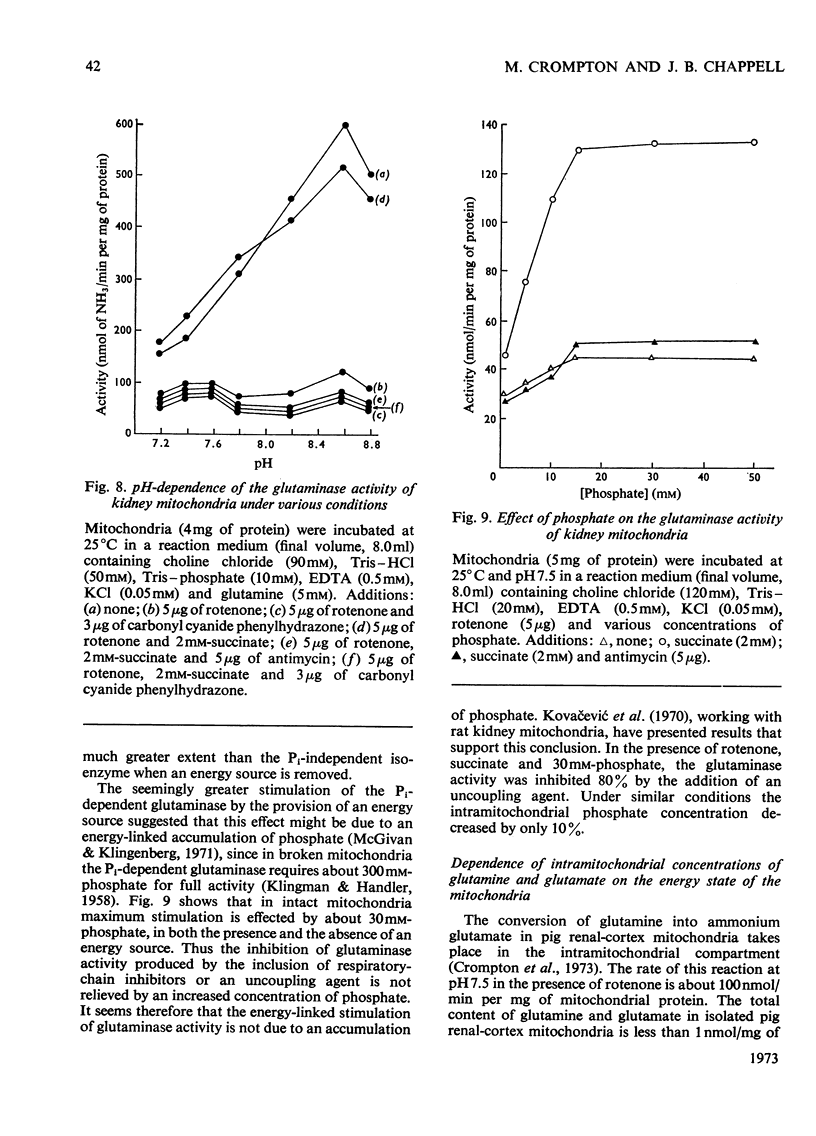
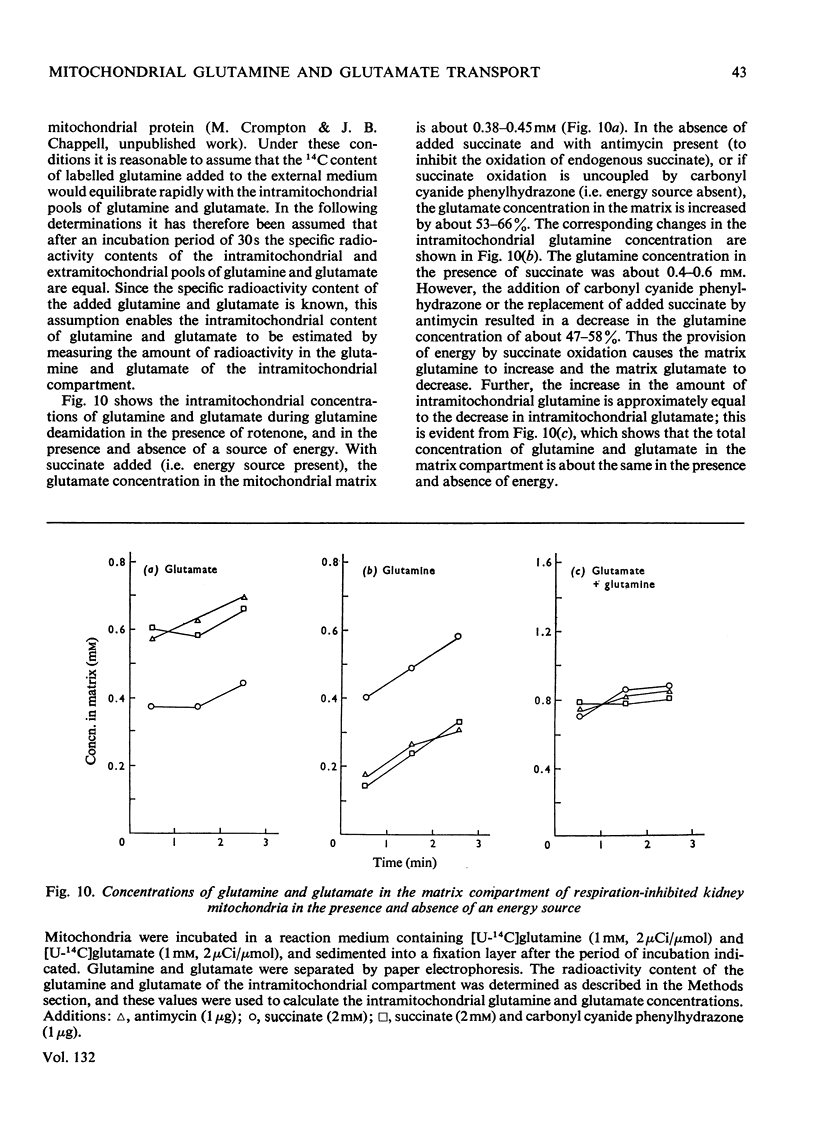
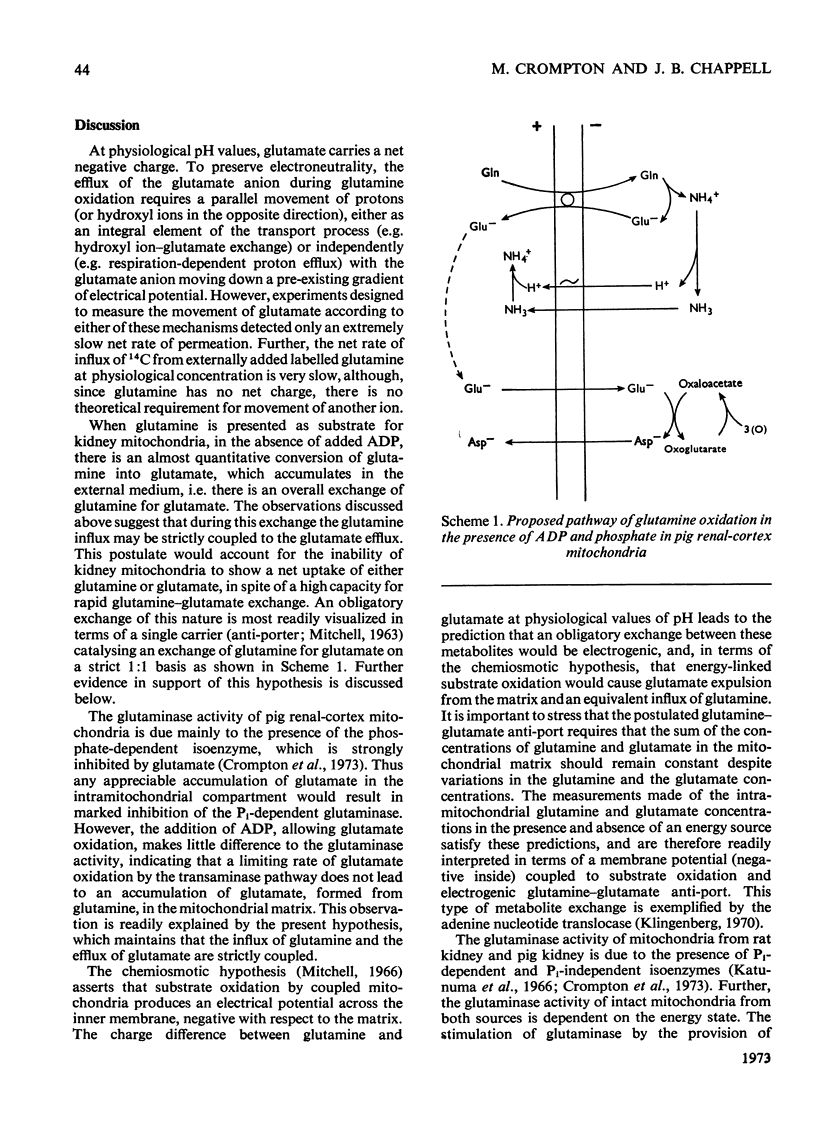
1. In the absence of added ADP glutamine is transformed by pig kidney mitochondria to ammonium glutamate, which appears in the external medium. This reaction is stimulated only slightly by the addition of ADP, but under these conditions about 20% of the glutamate is oxidized to aspartate. 2. Externally added glutamate is oxidized to aspartate, and at about the same rate as glutamine. 3. The net rates of glutamine and glutamate influx into the intramitochondrial compartment are very slow. 4. The phosphate-dependent glutaminase activity of intact mitochondria is stimulated by the provision of energy. 5. The provision of energy also decreases the concentration of glutamate and increases the concentration of glutamine in the intramitochondrial compartment. These energy-linked changes in the glutamine and glutamate concentrations are of equal magnitude. 6. It is suggested that transport of glutamine and glutamate across the inner membrane of kidney mitochondria occurs by an obligatory exchange between the two metabolites, and is electrogenic. The existence of an electrogenic glutamine–glutamate anti-porter is proposed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORST P., SLATER E. C. The oxidation of glutamate by rat-heart sarcosomes. Biochim Biophys Acta. 1960 Jun 17;41:170–171. doi: 10.1016/0006-3002(60)90391-7. [DOI] [PubMed] [Google Scholar]
- BORST P. The pathway of glutamate oxidation by mitochondria isolated from different tissues. Biochim Biophys Acta. 1962 Feb 26;57:256–269. doi: 10.1016/0006-3002(62)91119-8. [DOI] [PubMed] [Google Scholar]
- BRAUNSTEIN A. E. Les voices principales de l'assimilation et dissimilation de l'azote chez les animaux. Adv Enzymol Relat Subj Biochem. 1957;19:335–389. [PubMed] [Google Scholar]
- Chappell J. B. Systems used for the transport of substrates into mitochondria. Br Med Bull. 1968 May;24(2):150–157. doi: 10.1093/oxfordjournals.bmb.a070618. [DOI] [PubMed] [Google Scholar]
- Chappell J. B. The oxidation of citrate, isocitrate and cis-aconitate by isolated mitochondria. Biochem J. 1964 Feb;90(2):225–237. doi: 10.1042/bj0900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M., McGivan J. D., Chappell J. B. The intramitochondrial location of the glutaminase isoenzymes of pig kidney. Biochem J. 1973 Jan;132(1):27–34. doi: 10.1042/bj1320027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson P. J., McGivan J. D., Chappell J. B. The action of certain antibiotics on mitochondrial, erythrocyte and artificial phospholipid membranes. The role of induced proton permeability. Biochem J. 1969 Feb;111(4):521–535. doi: 10.1042/bj1110521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hird F. J., Marginson M. A. The formation of ammonia from glutamine and glutamate by mitochondria from rat liver and kidney. Arch Biochem Biophys. 1968 Sep 20;127(1):718–724. doi: 10.1016/0003-9861(68)90282-8. [DOI] [PubMed] [Google Scholar]
- KLINGMAN J. D., HANDLER P. Partial purification and properties of renal glutaminase. J Biol Chem. 1958 May;232(1):369–380. [PubMed] [Google Scholar]
- KREBS H. A., BELLAMY D. The interconversion of glutamic acid and aspartic acid in respiring tissues. Biochem J. 1960 Jun;75:523–529. doi: 10.1042/bj0750523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUN E., AYLING J. E., BALTIMORE B. G. STUDIES ON SPECIFIC ENZYME INHIBITORS. 8. ENZYME-REGULATORY MECHANISM OF THE ENTRY OF GLUTAMIC ACID INTO METABOLIC PATHWAYS IN KIDNEY TISSUE. J Biol Chem. 1964 Sep;239:2896–2904. [PubMed] [Google Scholar]
- Katunuma N., Tomino I., Nishino H. Glutaminase isozymes in rat kidney. Biochem Biophys Res Commun. 1966 Feb 3;22(3):321–328. doi: 10.1016/0006-291x(66)90485-2. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Metabolite transport in mitochondria: an example for intracellular membrane function. Essays Biochem. 1970;6:119–159. [PubMed] [Google Scholar]
- Kovacević Z., McGivan J. D., Chappell J. B. Conditions for activity of glutaminase in kidney mitochondria. Biochem J. 1970 Jun;118(2):265–274. doi: 10.1042/bj1180265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGivan J. D., Klingenberg M. Correlation between H+ and anion movement in mitochondria and the key role of the phosphate carrier. Eur J Biochem. 1971 Jun 11;20(3):392–399. doi: 10.1111/j.1432-1033.1971.tb01405.x. [DOI] [PubMed] [Google Scholar]
- Peterson P. J. Separation of acidic amino acids by high voltage paper electrophoresis and paper chromatography. J Chromatogr. 1968 Nov 19;38(2):301–303. doi: 10.1016/0021-9673(68)85045-9. [DOI] [PubMed] [Google Scholar]
- WERKHEISER W. C., BARTLEY W. The study of steady-state concentrations of internal solutes of mitochondria by rapid centrifugal transfer to a fixation medium. Biochem J. 1957 May;66(1):79–91. doi: 10.1042/bj0660079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU C. GLUTAMINE SYNTHETASE. I. A COMPARATIVE STUDY OF ITS DISTRIBUTION IN ANIMALS AND ITS INHIBITION BY DL-ALLO-DELTA-HYDROXYLYSINE. Comp Biochem Physiol. 1963 Apr;9:335–351. doi: 10.1016/0010-406x(63)90169-5. [DOI] [PubMed] [Google Scholar]
- WU C. GLUTAMINE SYNTHETASE. II. THE INTRACELLULAR LOCALIZATION IN THE RAT LIVER. Biochim Biophys Acta. 1963 Nov 8;77:482–493. doi: 10.1016/0006-3002(63)90524-9. [DOI] [PubMed] [Google Scholar]


