Abstract
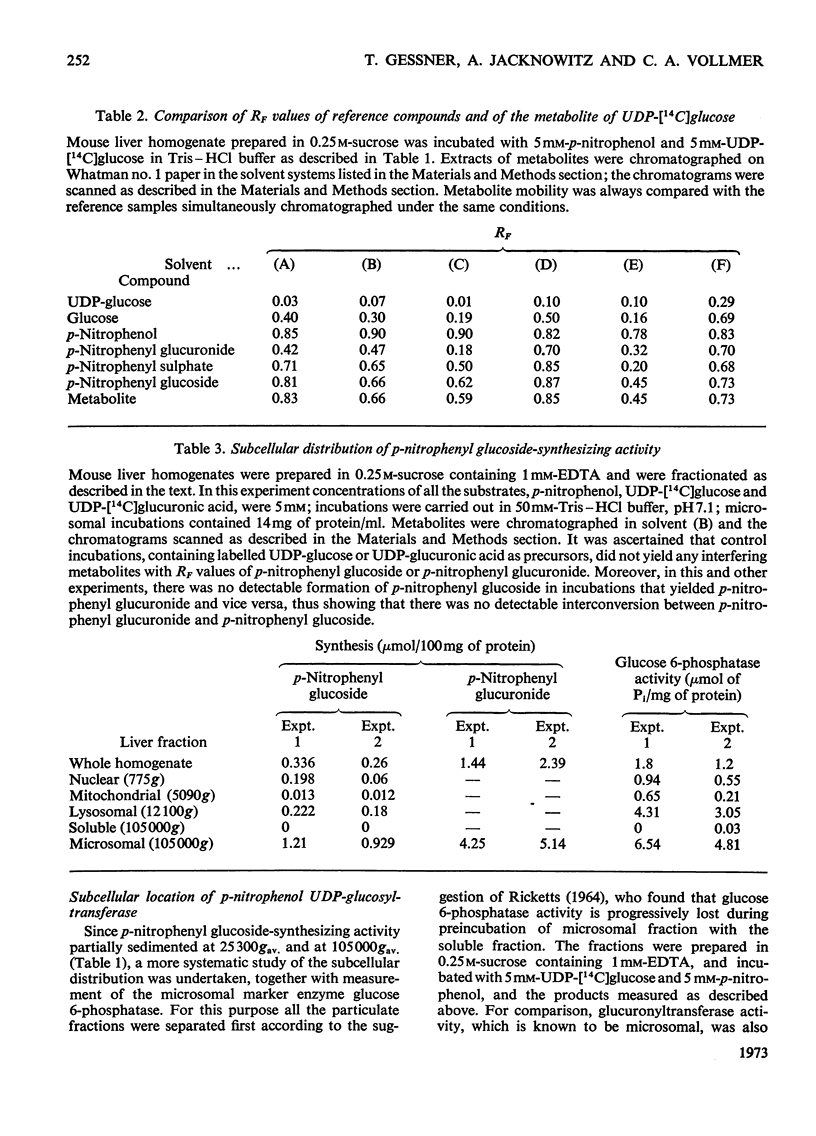
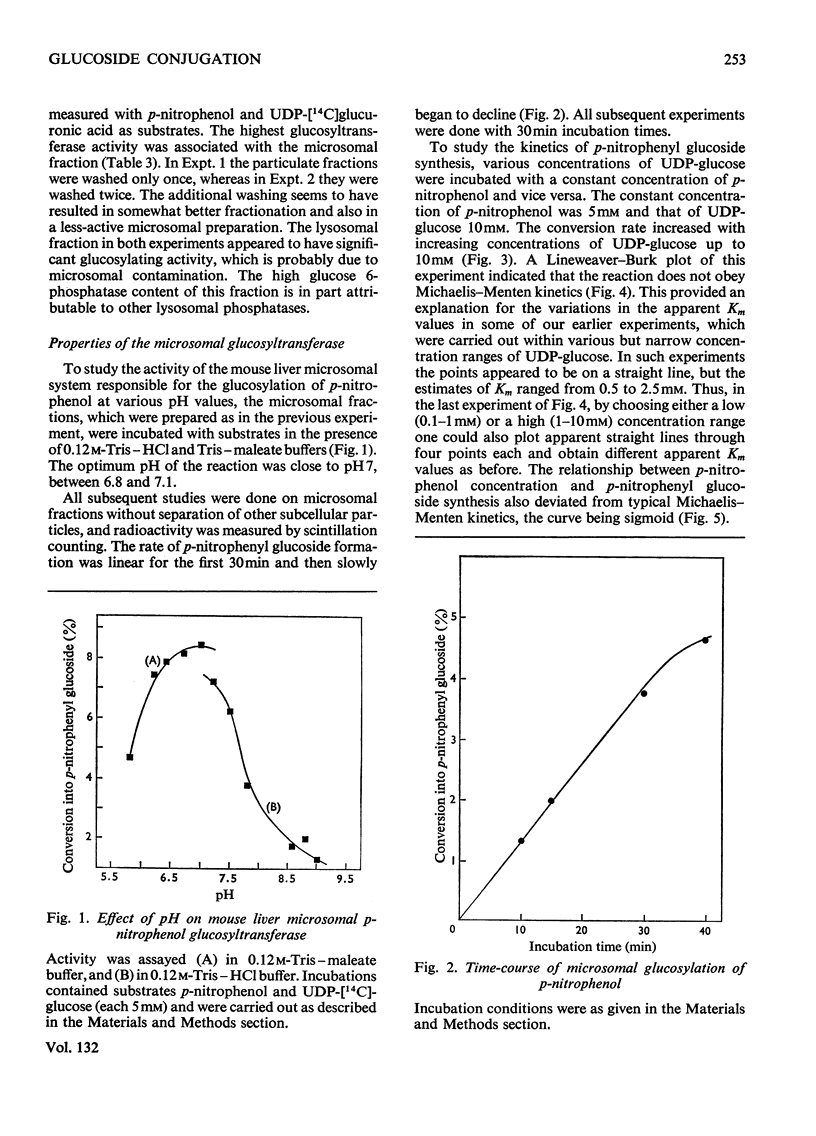
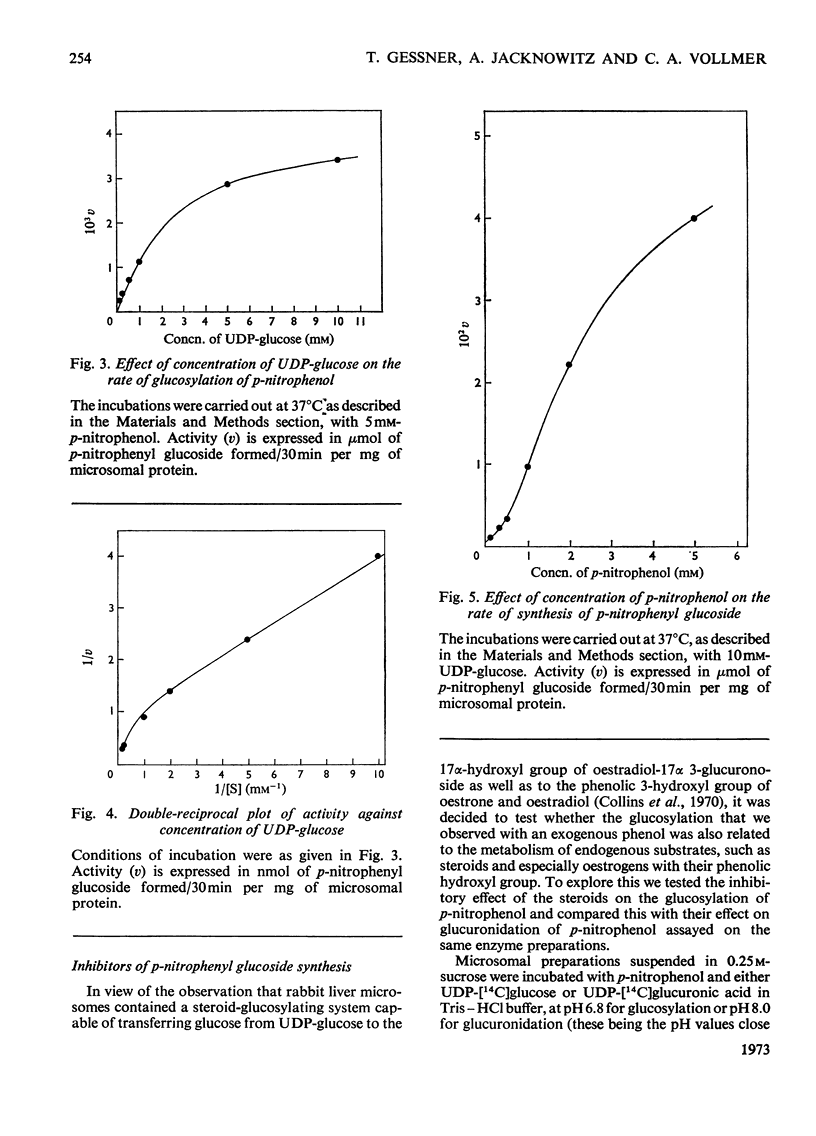
The mammalian glucoside-conjugation pathway was studied by using p-nitrophenol as the model substrate and mouse liver microsomal preparations as the source of enzyme. The microsomal preparations supplemented with UDP-glucose glucosylated p-nitrophenol; p-nitrophenyl glucoside was identified by chromatography in six solvent systems. The unsolubilized glucosyltransferase of fresh microsomal preparations did not follow the usual Michaelis–Menten kinetics and was easily inhibited by many steroids. All the steroids tested inhibited glucosylation of p-nitrophenol to a greater degree than glucuronidation of p-nitrophenol when assayed in the same microsomal preparations. The steroids inhibited glucosylation with the following decreasing effectiveness: pregnan-3α-ol-20β-one (3α-hydroxypregnan-20-β-one)>oestradiol-17β 3-methyl ether>oestradiol-17β>oestriol>pregnane-3α,20β-diol>oestrone. Pregnan-3α-ol-20β-one, pregnane-3α,20β-diol and oestrone had negligible effect on glucuronidation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARIAS I. M., GARTNER L. M., SEIFTER S., FURMAN M. PROLONGED NEONATAL UNCONJUGATED HYPERBILIRUBINEMIA ASSOCIATED WITH BREAST FEEDING AND A STEROID, PREGNANE-3(ALPHA), 20(BETA)-DIOL, IN MATERNAL MILK THAT INHIBITS GLUCURONIDE FORMATION IN VITRO. J Clin Invest. 1964 Nov;43:2037–2047. doi: 10.1172/JCI105078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlard B. P., Lathe G. H. Breast milk jaundice: effect of 3-alpha 20-beta-pregnanediol on bilirubin conjugation by human liver. Arch Dis Child. 1970 Apr;45(240):186–189. doi: 10.1136/adc.45.240.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlard B. P., Lathe G. H. The effect of steroids and nucleotidesoon solubilized bilirubin uridine diphosphate-glucuronyltransferase. Biochem J. 1970 Sep;119(3):437–445. doi: 10.1042/bj1190437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan B. R., Holton J. B., Lathe G. H. The effect of pregnanediol and pregnanediol glucuronide on bilirubin conjugation by rat liver slices. Clin Sci. 1965 Oct;29(2):353–361. [PubMed] [Google Scholar]
- Collins D. C., Jirku H., Layne D. S. Steroid N-acetylglucosaminyl transferase. Localization and some properties of the enzyme in rabbit tissues. J Biol Chem. 1968 Jun 10;243(11):2928–2933. [PubMed] [Google Scholar]
- Collins D. C., Williamson D. G., Layne D. S. Steroid glucosides. Enzymatic synthesis by a partially purified transferase from rabbit liver microsomes. J Biol Chem. 1970 Feb 25;245(4):873–876. [PubMed] [Google Scholar]
- Compernolle F., Van Hees G. P., Fevery J., Heirwegh K. P. Mass-spectrometric structure elucidation of dog bile azopigments as the acyl glycosides of glucopyranose and xylopyranose. Biochem J. 1971 Dec;125(3):811–819. doi: 10.1042/bj1250811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUTTON G. J. Uridine diphosphate glucuronic acid as glucuronyl donor in the synthesis of ester, aliphatic and steroid glucuronides. Biochem J. 1956 Dec;64(4):693–701. doi: 10.1042/bj0640693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton G. J. Uridine diphosphate glucose and the synthesis of phenolic glucosides by mollusks. Arch Biochem Biophys. 1966 Sep 26;116(1):399–405. doi: 10.1016/0003-9861(66)90046-4. [DOI] [PubMed] [Google Scholar]
- Fevery J., Van Hees G. P., Leroy P., Compernolle F., Heirwegh K. P. Excretion in dog bile of glucose and xylose conjugates of bilirubin. Biochem J. 1971 Dec;125(3):803–810. doi: 10.1042/bj1250803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. ASPARTATE TRANSCARBAMYLASE, AN ENZYME DESIGNED FOR FEEDBACK INHIBITION. Fed Proc. 1964 May-Jun;23:727–735. [PubMed] [Google Scholar]
- Gessner T., Acara M. Metabolism of thiols. S-Glucosylation. J Biol Chem. 1968 Jun 10;243(11):3142–3147. [PubMed] [Google Scholar]
- Gessner T., Hamada N. Identification of p-nitrophenyl glucoside as a urinary metabolite. J Pharm Sci. 1970 Oct;59(10):1528–1529. doi: 10.1002/jps.2600591043. [DOI] [PubMed] [Google Scholar]
- HSIA D. Y., DOWBEN R. M., SHAW R., GROSSMAN A. Inhibition of glucuronosyl transferase by progestational agents from serum of pregnant women. Nature. 1960 Aug 20;187:693–694. doi: 10.1038/187693a0. [DOI] [PubMed] [Google Scholar]
- Hargreaves T., Piper R. F. Breast milk jaundice. Effect of inhibitory breast milk and 3 alpha, 20 abeta-pregnanediol on glucuronyl transferase. Arch Dis Child. 1971 Apr;46(246):195–198. doi: 10.1136/adc.46.246.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES B. GLUCURONYL TRANSFERASE INHIBITION BY STEROIDS. J Pediatr. 1964 Jun;64:815–821. doi: 10.1016/s0022-3476(64)80639-9. [DOI] [PubMed] [Google Scholar]
- Krauer-Mayer B., Keller M., Hottinger A. Uber den frauenmilchinduzierten Icterus prolongatus des Neugeborenen. Helv Paediatr Acta. 1968 Feb;23(1):68–76. [PubMed] [Google Scholar]
- Kuenzle C. C. Bilirubin conjugates of human bile. Isolation of phenylazo derivatives of bile bilirubin. Biochem J. 1970 Sep;119(3):387–394. doi: 10.1042/bj1190387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzle C. C. Bilirubin conjugates of human bile. Nuclear-magnetic-resonance, infrared and optical spectra of model compounds. Biochem J. 1970 Sep;119(3):395–409. doi: 10.1042/bj1190395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzle C. C. Bilirubin conjugates of human bile. The excretion of bilirubin as the acyl glycosides of aldobiouronic acid, pseudoaldobiouronic acid and hexuronosylhexuronic acid, with a branched-chain hexuronic acid as one of the components of the hexuronosylhexuronide. Biochem J. 1970 Sep;119(3):411–435. doi: 10.1042/bj1190411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LATHE G. H., WALKER M. Inhibition of bilirubin conjugation in rat liver slices by human pregnancy and neonatal serum and steroids. Q J Exp Physiol Cogn Med Sci. 1958 Jul;43(3):257–265. doi: 10.1113/expphysiol.1958.sp001328. [DOI] [PubMed] [Google Scholar]
- LELOIR L. F., GOLDEMBERG S. H. Synthesis of glycogen from uridine diphosphate glucose in liver. J Biol Chem. 1960 Apr;235:919–923. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levy G., Procknal J. A. Drug biotransformation interactions in man. I. Mutual inhibition in glucuronide formation of salicylic acid and salicylamide in man. J Pharm Sci. 1968 Aug;57(8):1330–1335. doi: 10.1002/jps.2600570811. [DOI] [PubMed] [Google Scholar]
- MONOD J., CHANGEUX J. P., JACOB F. Allosteric proteins and cellular control systems. J Mol Biol. 1963 Apr;6:306–329. doi: 10.1016/s0022-2836(63)80091-1. [DOI] [PubMed] [Google Scholar]
- RICKETTS T. R. A CELL SAP INHIBITOR OF ENZYMIC ACTIVITY COMPLICATING DIFFERENTIAL CENTRIFUGATION STUDIES OF ENZYME LOCATION. Exp Cell Res. 1964 May;34:557–567. doi: 10.1016/0014-4827(64)90242-3. [DOI] [PubMed] [Google Scholar]
- Rosenfeld R. S., Arias I. M., Gartner L. M., Hellman L., Gallagher T. F. Studies of urinary pregnane-3 alpha,20 beta-diol during pregnancy, post-partum, lactation and progesterone ingestion. J Clin Endocrinol Metab. 1967 Dec;27(12):1705–1710. doi: 10.1210/jcem-27-12-1705. [DOI] [PubMed] [Google Scholar]
- Severi F., Rondini G., Zaverio S., Vegni M. Prolonged neonatal hyperbilirubinemia and pregnane-3(alpha),20(beta)-diol in maternal milk. Helv Paediatr Acta. 1970 Nov;25(5):517–521. [PubMed] [Google Scholar]
- Smith J. N. The comparative metabolism of xenobiotics. Adv Comp Physiol Biochem. 1968;3:173–232. doi: 10.1016/b978-0-12-395512-8.50009-9. [DOI] [PubMed] [Google Scholar]
- Williamson D. G., Collins D. C., Layne D. S., Conrow R. B., Bernstein S. Isolation of 17-alpha-estradiol 17-beta-D-glucopyranoside from rabbit urine, and its synthesis and characterization. Biochemistry. 1969 Nov;8(11):4299–4304. doi: 10.1021/bi00839a012. [DOI] [PubMed] [Google Scholar]
- Williamson D. G., Polakova A., Layne D. S. Estrogen glucosides and galactosides: formation by rabbit liver microsomes in vitro. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1057–1062. doi: 10.1016/0006-291x(71)90011-8. [DOI] [PubMed] [Google Scholar]
- Winsnes A. Studies on the activation in vitro of glucuronyltransferase. Biochim Biophys Acta. 1969 Nov 4;191(2):279–291. doi: 10.1016/0005-2744(69)90247-2. [DOI] [PubMed] [Google Scholar]
- Wong K. P. Formation of bilirubin glucoside. Biochem J. 1971 Dec;125(4):929–934. doi: 10.1042/bj1250929a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y. K., Wood B. S. Breast-milk jaundice and oral contraceptives. Br Med J. 1971 Nov 13;4(5784):403–404. doi: 10.1136/bmj.4.5784.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAHA T., CARDINI C. E. The biosynthesis of plant glycosides. I. Monoglucosides. Arch Biochem Biophys. 1960 Jan;86:127–132. doi: 10.1016/0003-9861(60)90379-9. [DOI] [PubMed] [Google Scholar]


