Abstract
1. A special population of rough microsomal material (microsomes) rich in lysosomal acid hydrolases was separated by isopycnic centrifugation as a discrete fraction (RM2) from the bulk of rough microsomal material in rat kidney because of its greater density. 2. The specific activities of five acid hydrolases in the RM2 fraction were approximately one-half those of a purified lysosomal (L) fraction and 10- to 30-fold greater than those of an ordinary rough microsomal (RM1) fraction. 3. These special rough microsomes have a distinctive ultrastructure and electron-cytochemical properties. Their cisternal content resembles the matrix of lysosomes in that it is electron-dense, osmiophilic and plumbophilic and gives a positive reaction for acid phosphatase activity. 4. Polyacrylamide-gel electrophoresis of soluble proteins from the L fraction resolved nine anionic glycoproteins, most of which exhibit acid hydrolase activities (Goldstone & Koenig, 1970, 1973; Goldstone et al., 1971a). The most anionic glycoprotein is the acidic lipoglycoprotein of the lysosomal matrix (Goldstone et al., 1970). 5. Polyacrylamide-gel electrophoresis of soluble proteins from the RM2 fraction resolved two cationic glycoproteins with acid hydrolase activities (Goldstone & Koenig, 1973) and an anionic glycoprotein with the same electrophoretic mobility as the lysosomal lipoglycoprotein, but without its lipid constituents or capacity to bind the basic fluorochrome Acridine Orange. These constituents are considered to be the precursors of the lysosomal glycoproteins.
Full text
PDF




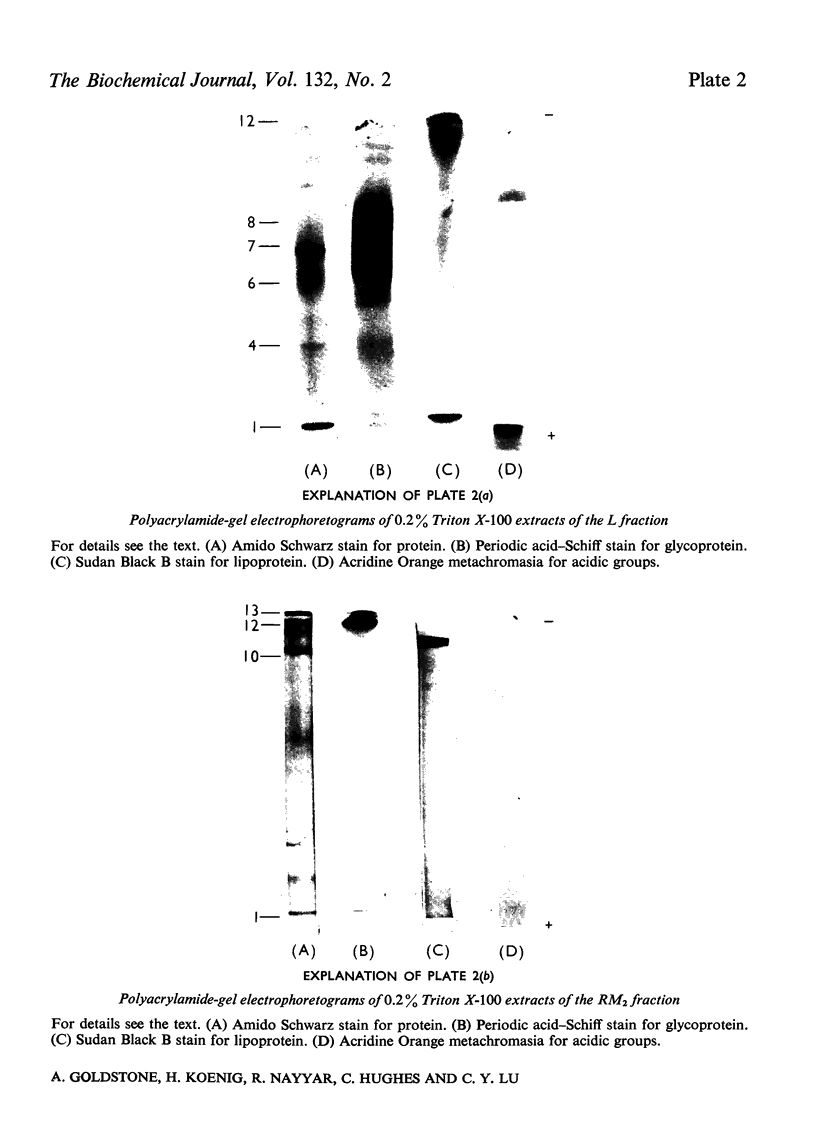
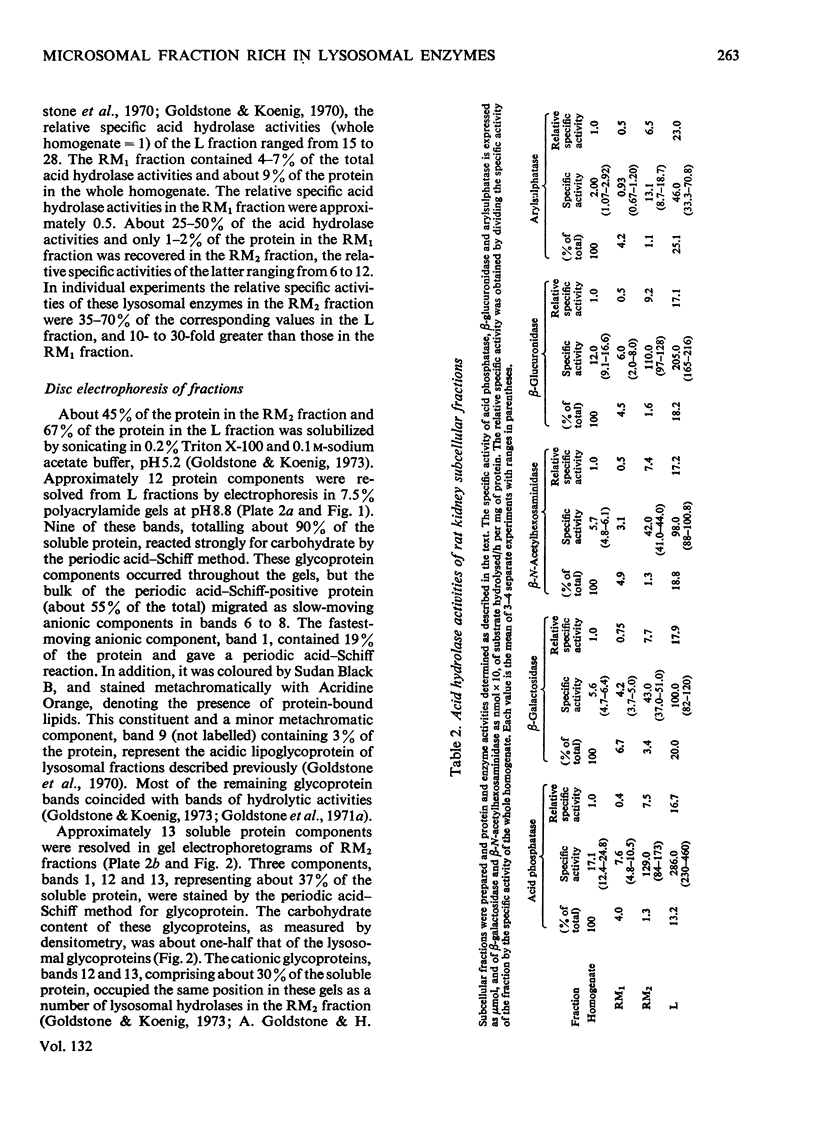
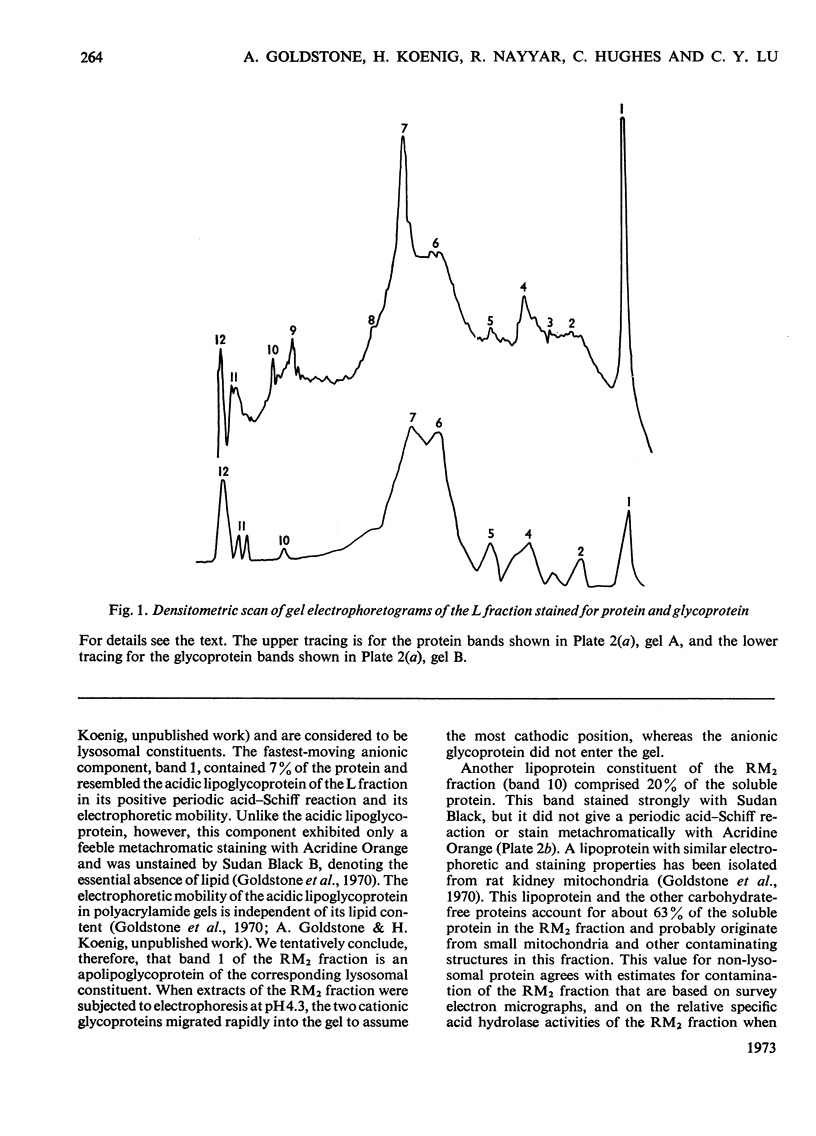
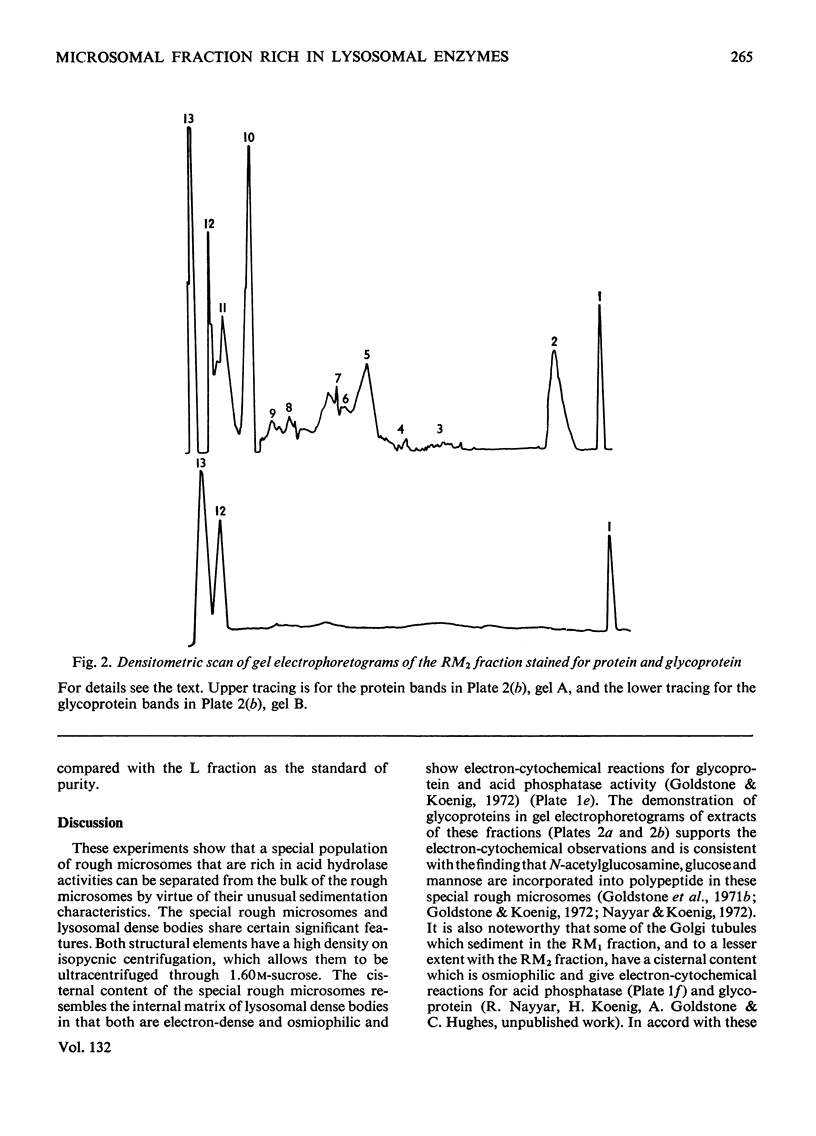

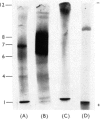
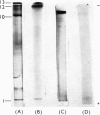
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- CARO L. G., PALADE G. E. PROTEIN SYNTHESIS, STORAGE, AND DISCHARGE IN THE PANCREATIC EXOCRINE CELL. AN AUTORADIOGRAPHIC STUDY. J Cell Biol. 1964 Mar;20:473–495. doi: 10.1083/jcb.20.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALLNER G., ORRENIUS S., BERGSTRAND A. Isolation and properties of rough and smooth vesicles from rat liver. J Cell Biol. 1963 Feb;16:426–430. doi: 10.1083/jcb.16.2.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., WATTIAUX R. Tissue fractionation studies. VII. Release of bound hydrolases by means of triton X-100. Biochem J. 1956 Aug;63(4):606–608. doi: 10.1042/bj0630606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallner G., Nilsson R. Mechanism of the cation effect in subfractionation of microsomes. J Cell Biol. 1966 Oct;31(1):181–193. doi: 10.1083/jcb.31.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- GIANETTO R., DE DUVE C. Tissue fractionation studies. 4. Comparative study of the binding of acid phosphatase, beta-glucuronidase and cathepsin by rat-liver particles. Biochem J. 1955 Mar;59(3):433–438. doi: 10.1042/bj0590433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone A., Koenig H. Lysosomal hydrolases as glycoproteins. Life Sci II. 1970 Dec 8;9(23):1341–1350. doi: 10.1016/0024-3205(70)90115-3. [DOI] [PubMed] [Google Scholar]
- Goldstone A., Koenig H. Physicochemical modifications of lysosomal hydrolases during intracellular transport. Biochem J. 1973 Feb;132(2):267–282. doi: 10.1042/bj1320267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone A., Konecny P., Koenig H. Lysosomal hydrolases: Conversion of acidic to basic forms by neuraminidase. FEBS Lett. 1971 Feb 12;13(1):68–72. doi: 10.1016/0014-5793(71)80667-1. [DOI] [PubMed] [Google Scholar]
- Goldstone A., Szabo E., Koenig H. Isolation and characterization of acidic lipoprotein in renal and hepatic lysosomes. Life Sci II. 1970 Jun 8;9(11):607–616. doi: 10.1016/0024-3205(70)90211-0. [DOI] [PubMed] [Google Scholar]
- HUTCHISON W. C., MUNRO H. N. The determination of nucleic acids in biological materials. A review. Analyst. 1961 Dec;86:768–813. doi: 10.1039/an9618600768. [DOI] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. II. Transport to condensing vacuoles and zymogen granules. J Cell Biol. 1967 Aug;34(2):597–615. doi: 10.1083/jcb.34.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOENIG H. Histological distribution of brain gangliosides: lysosomes as glycolipoprotein granules. Nature. 1962 Aug 25;195:782–784. doi: 10.1038/195782a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mego J. L. The effect of pH on cathepsin activities in mouse liver heterolysosomes. Biochem J. 1971 May;122(4):445–452. doi: 10.1042/bj1220445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NADLER N. J., YOUNG B. A., LEBLOND C. P., MITMAKER B. ELABORATION OF THYROGLOBULIN IN THE THYROID FOLLICLE. Endocrinology. 1964 Mar;74:333–354. doi: 10.1210/endo-74-3-333. [DOI] [PubMed] [Google Scholar]
- ROY A. B. Comparative studies on the liver sulphatases. Biochem J. 1958 Mar;68(3):519–528. doi: 10.1042/bj0680519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D., Price R. G., Dance N. Separation and properties of beta-galactosidase, beta-glucosidase, beta-glucuronidase and N-acetyl-beta-glucosaminidase from rat kidney. Biochem J. 1967 Feb;102(2):525–532. doi: 10.1042/bj1020525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter H., Jabbal I., Hudgin R. L., Pinteric L., McGuire E. J., Roseman S. Intracellular localization of liver sugar nucleotide glycoprotein glycosyltransferases in a Golgi-rich fraction. J Biol Chem. 1970 Mar 10;245(5):1090–1100. [PubMed] [Google Scholar]
- Scott J. E., Harbinson R. J. Periodate oxidation of acid polysaccharides inhibition by the electrostatic field of the substrate. Histochemie. 1968;14(3):215–220. doi: 10.1007/BF00306317. [DOI] [PubMed] [Google Scholar]