Abstract
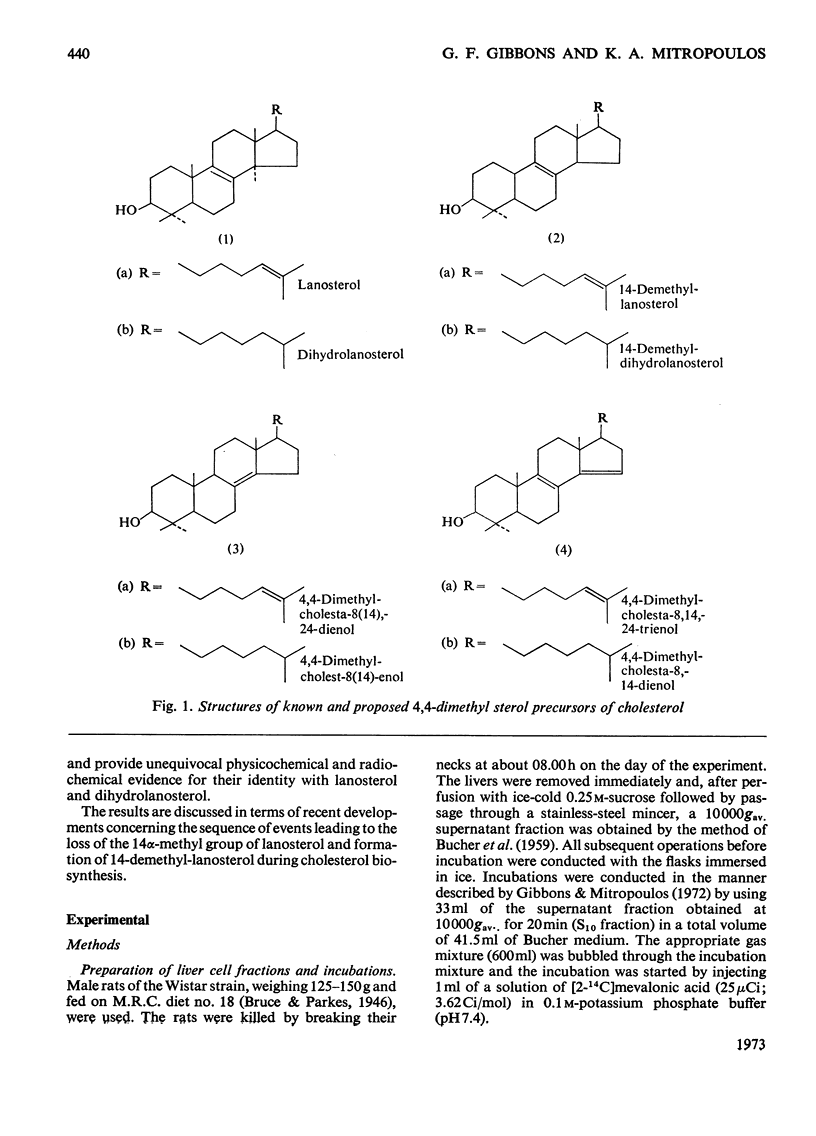
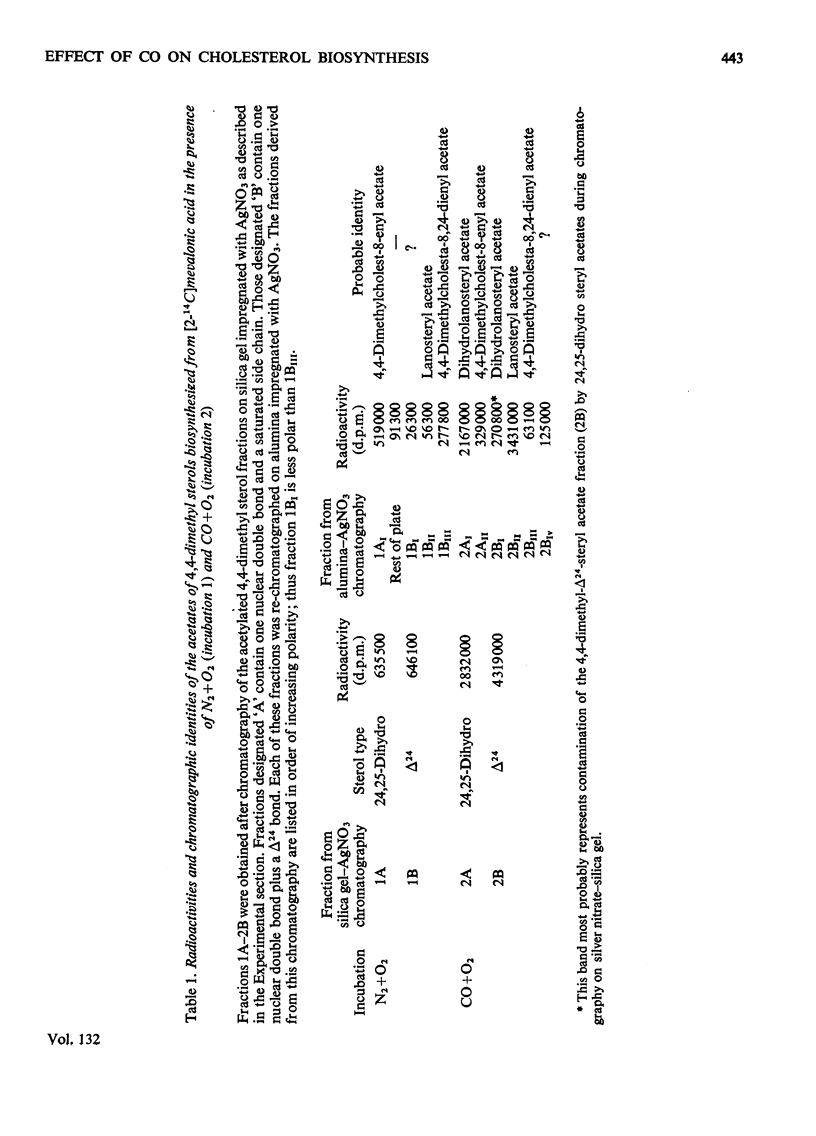
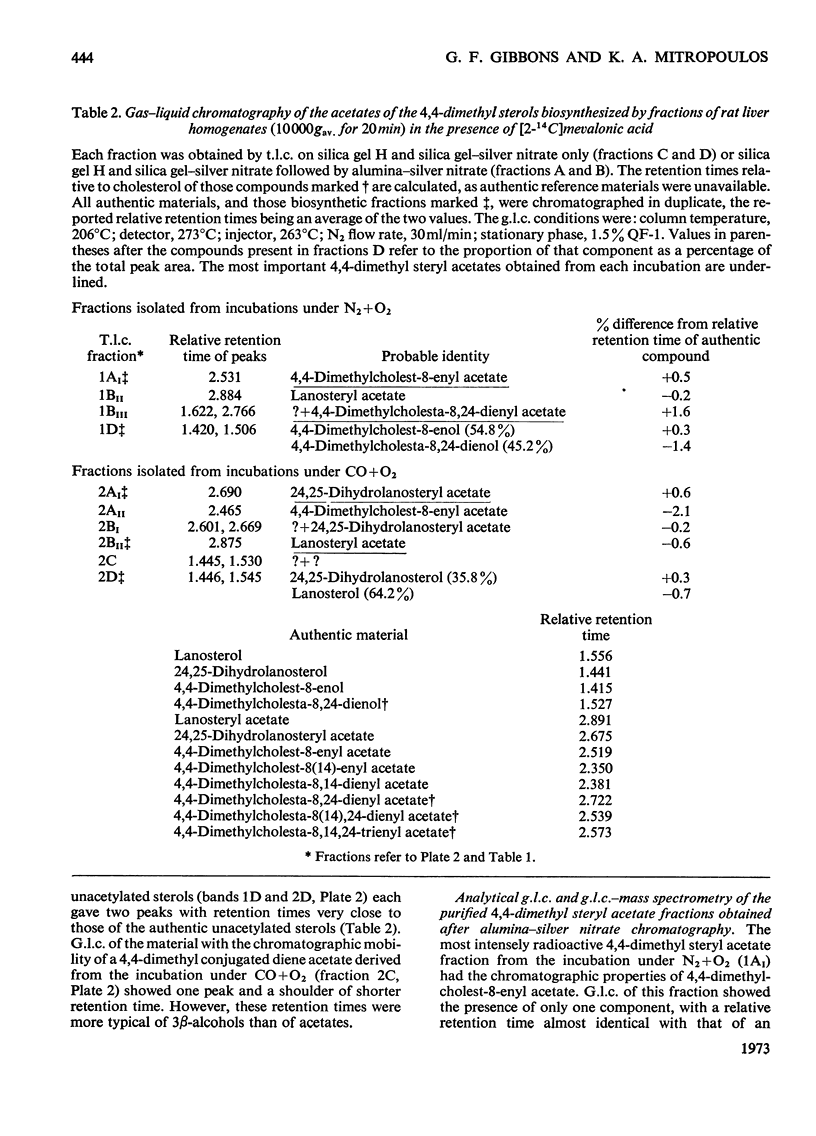
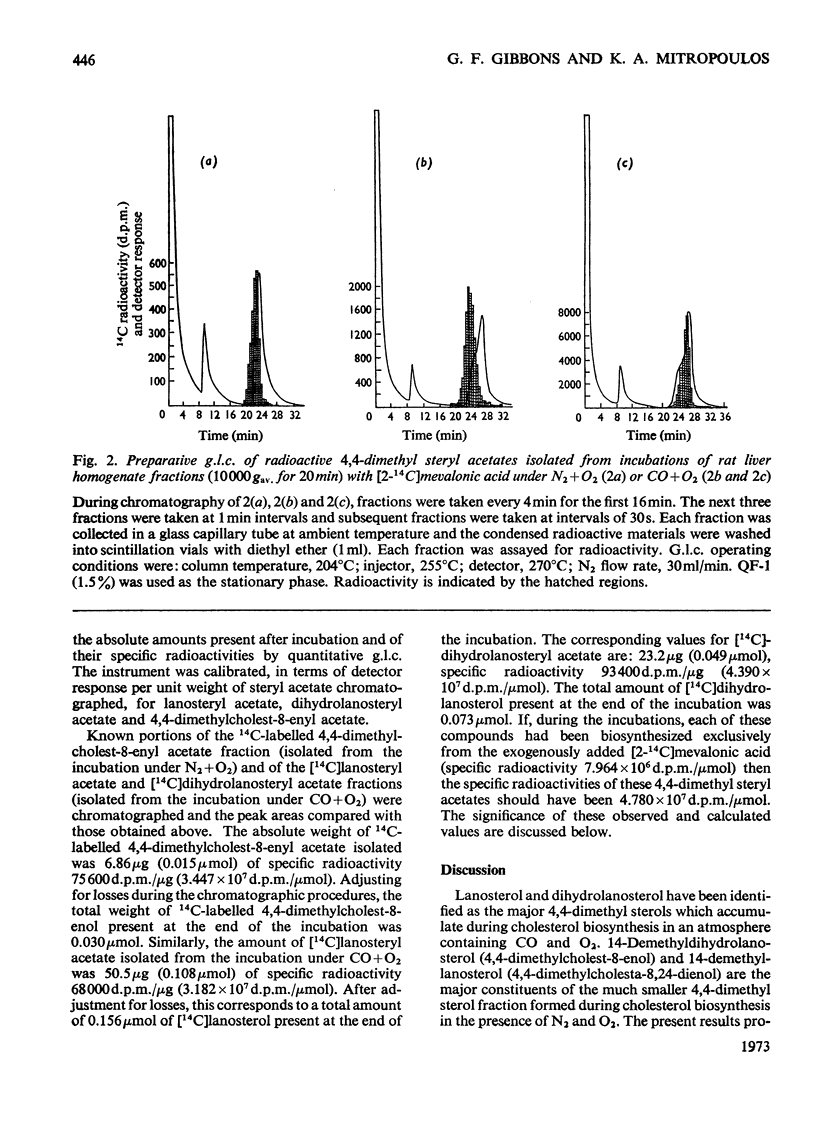
Cholesterol biosynthesis was studied in rat liver subcellular fractions incubated with dl-[2-14C]mevalonic acid under gas phases consisting of either N2+O2 (90:10) or CO+O2 (90:10). CO inhibits cholesterol biosynthesis from [2-14C]mevalonic acid and results in a large accumulation of radioactive 4,4-dimethyl sterols. Separation of the components of the 4,4-dimethyl sterol fraction showed that lanosterol and dihydrolanosterol are the major components that accumulate during cholesterol biosynthesis in an atmosphere containing CO, whereas 14-demethyl-lanosterol and 14-demethyldihydrolanosterol are the major components of the much less intensely radioactive 4,4-dimethyl sterol fraction isolated from incubations with N2+O2 as the gas phase. The identities of lanosterol, dihydrolanosterol and 14-demethyldihydrolanosterol were confirmed by both radiochemical and physicochemical methods, including g.l.c. and combined g.l.c.–mass spectrometry. CO therefore results in a qualitative as well as a quantitative difference in the 4,4-dimethyl sterol fraction which arises during cholesterol biosynthesis from mevalonic acid. The specific radioactivity of the [14C]lanosterol biosynthesized in the presence of CO was lower than that of its companion, [14C]dihydrolanosterol. The relative amounts of 4,4-dimethyl-Δ24-sterols and 4,4-dimethyl-24,25-dihydrosterols present in each type of incubation suggest that enzymic reduction of the sterol side chain occurs predominantly at a stage after that of lanosterol.
Full text
PDF







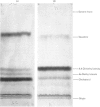
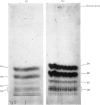
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhtar M., Wilton D. C., Watkinson I. A., Rahimtula A. D. Substrate activation in pyridine nucleotide-linked reactions: illustrations from the steroid field. Proc R Soc Lond B Biol Sci. 1972 Feb 15;180(1059):167–177. doi: 10.1098/rspb.1972.0012. [DOI] [PubMed] [Google Scholar]
- BUCHER N. L., McGARRAHAN K., GOULD E., LOUD A. V. Cholesterol biosynthesis in preparations of liver from normal, fasting, x-irradiated, cholesterol-fed, triton, or delta 4-cholesten-3-one-treated rats. J Biol Chem. 1959 Feb;234(2):262–267. [PubMed] [Google Scholar]
- CLAYTON R. B., NELSON A. N., FRANTZ I. D., Jr THE SKIN STEROLS OF NORMAL AND TRIPARANOL-TREATED RATS. J Lipid Res. 1963 Apr;4:166–176. [PubMed] [Google Scholar]
- Fiecchi A., GAlli Kienle M., Scala A., Galli G., Grossi Paoletti E., Cattabeni F., Paoletti R. Hydrogen exchange and double bond formation in cholesterol biosynthesis. Proc R Soc Lond B Biol Sci. 1972 Feb 15;180(1059):147–165. doi: 10.1098/rspb.1972.0011. [DOI] [PubMed] [Google Scholar]
- GAUTSCHI F., BLOCH K. Synthesis of isomeric 4,4-dimethylcholestenols and identification of a lanosterol metabolite. J Biol Chem. 1958 Dec;233(6):1343–1347. [PubMed] [Google Scholar]
- Galli G., Maroni S. Mass spectrometric investigations of some unsaturated sterols biosynthetically related to cholesterol. Steroids. 1967 Sep;10(3):189–197. doi: 10.1016/0039-128x(67)90046-3. [DOI] [PubMed] [Google Scholar]
- Gibbons G. F., Mitropoulos K. A. Inhibition of cholesterol biosynthesis by carbon monoxide: accumulation of lanosterol and 24,25-dihydrolanosterol. Biochem J. 1972 Mar;127(1):315–317. doi: 10.1042/bj1270315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLSON J. A., Jr, LINDBERG M., BLOCH K. On the demethylation of lanosterol to cholesterol. J Biol Chem. 1957 Jun;226(2):941–956. [PubMed] [Google Scholar]
- POPJAK G. Biosynthesis of squalene and cholesterol in vitro from acetate-1-C14. Arch Biochem Biophys. 1954 Jan;48(1):102–106. doi: 10.1016/0003-9861(54)90310-0. [DOI] [PubMed] [Google Scholar]
- Paliokas A. M., Schroepfer G. J., Jr Stereospecificity in the enzymatic conversion of delta-7-cholesten-3-beta-ol to 7-dehydrocholesterol. J Biol Chem. 1968 Feb 10;243(3):453–464. [PubMed] [Google Scholar]
- Scallen T. J., Dhar A. K., Loughran E. D. Isolation and characterization of C-4 methyl intermediates in cholesterol biosynthesis after treatment of rat liver in vitro with cholestan-3 beta, 5 alpha,6 beta-triol. J Biol Chem. 1971 May 25;246(10):3168–3174. [PubMed] [Google Scholar]
- Schroepfer G. J., Jr, Lutsky B. N., Martin J. A., Huntoon S., Fourcans B., Lee W. H., Vermilion J. Recent investigations on the nature of sterol intermediates in the biosynthesis of cholesterol. Proc R Soc Lond B Biol Sci. 1972 Feb 15;180(1059):125–146. [PubMed] [Google Scholar]
- Swindell A. C., Gaylor J. L. Investigation of the component reactions of oxidative sterol demethylation. Formation and metabolism of 3-ketosteroid intermediates. J Biol Chem. 1968 Nov 10;243(21):5546–5555. [PubMed] [Google Scholar]
- Watkinson I. A., Wilton D. C., Munday K. A., Akhtar M. The formation and reduction of the 14,15-double bond in cholesterol biosynthesis. Biochem J. 1971 Jan;121(1):131–137. doi: 10.1042/bj1210131. [DOI] [PMC free article] [PubMed] [Google Scholar]