Abstract
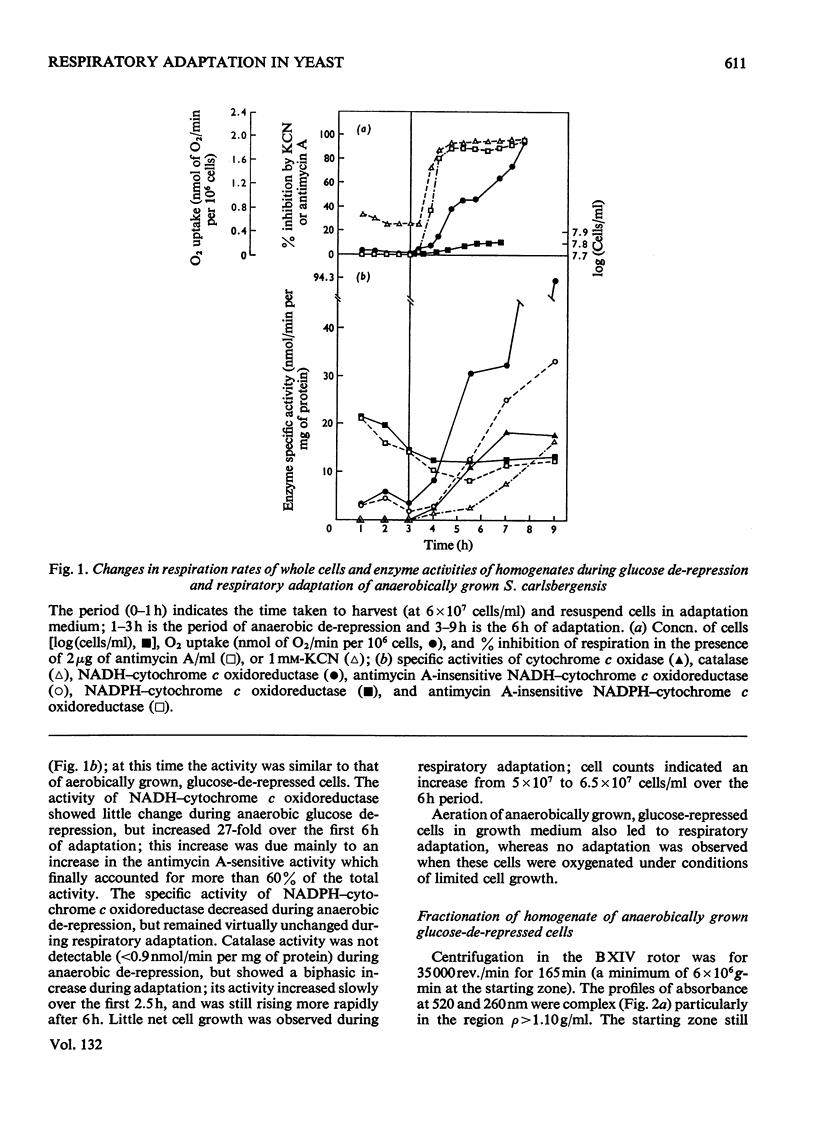
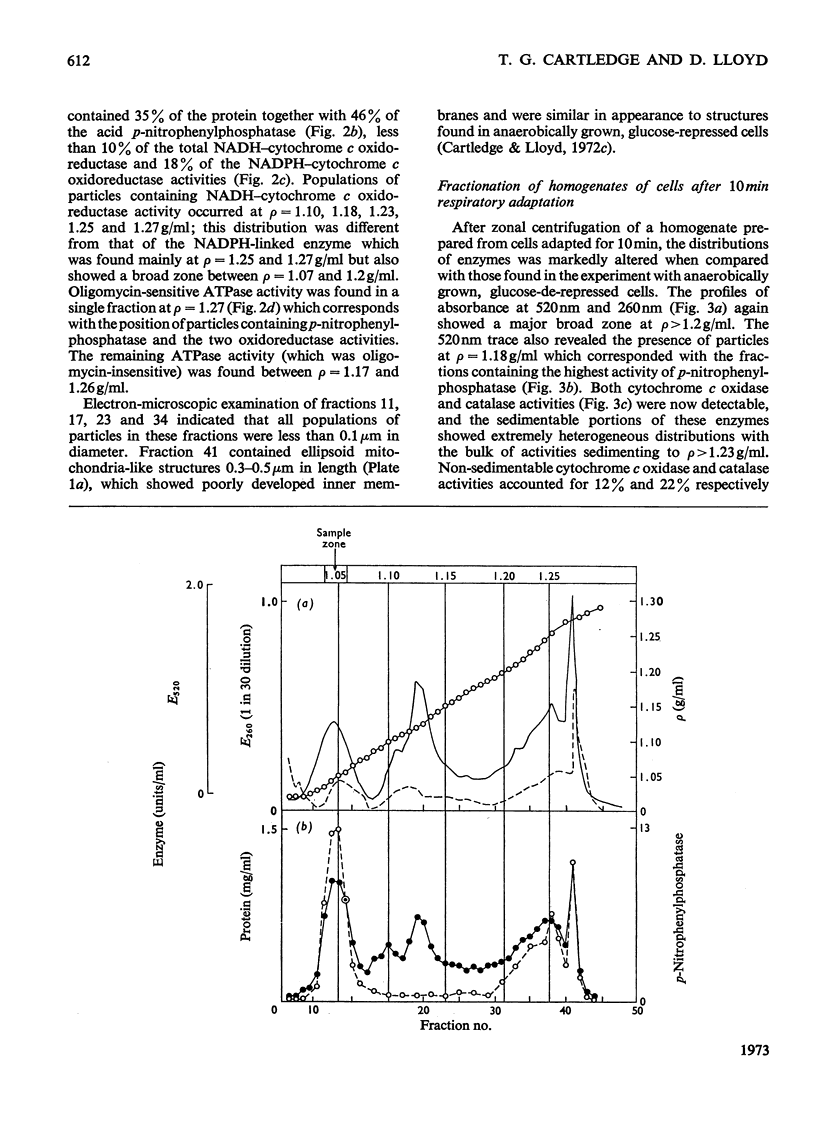
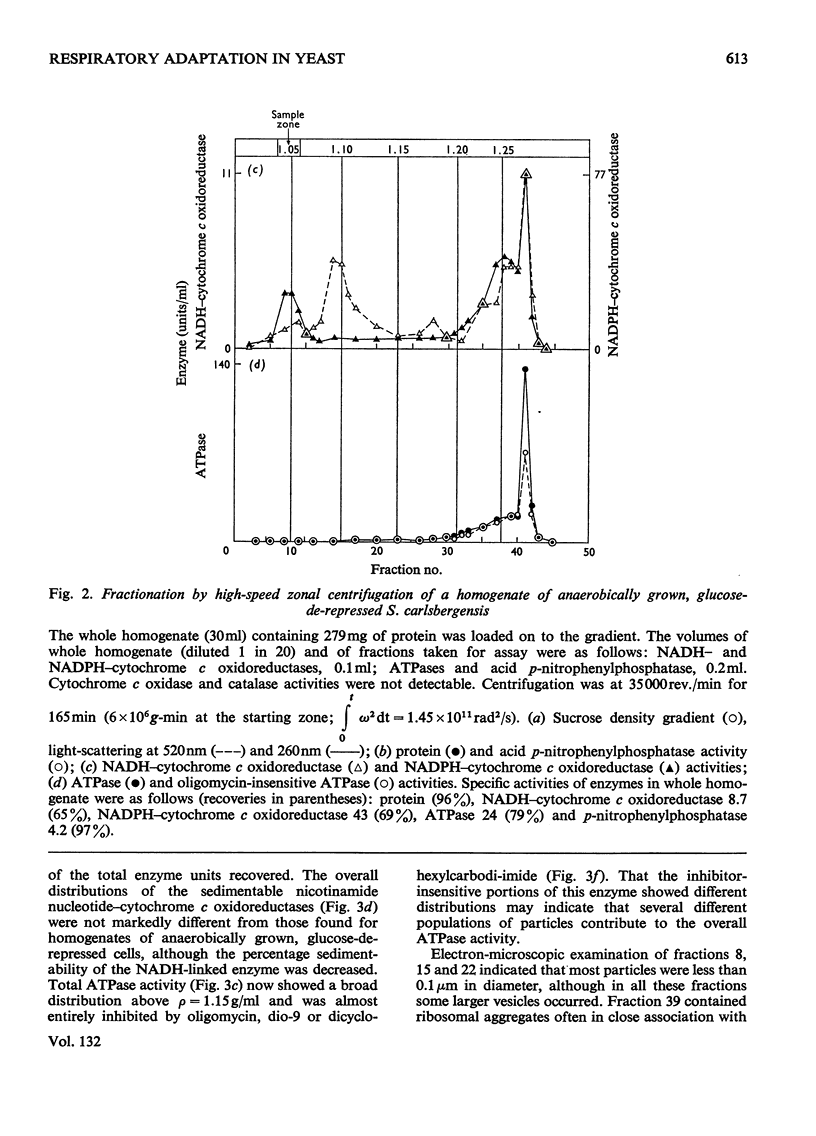
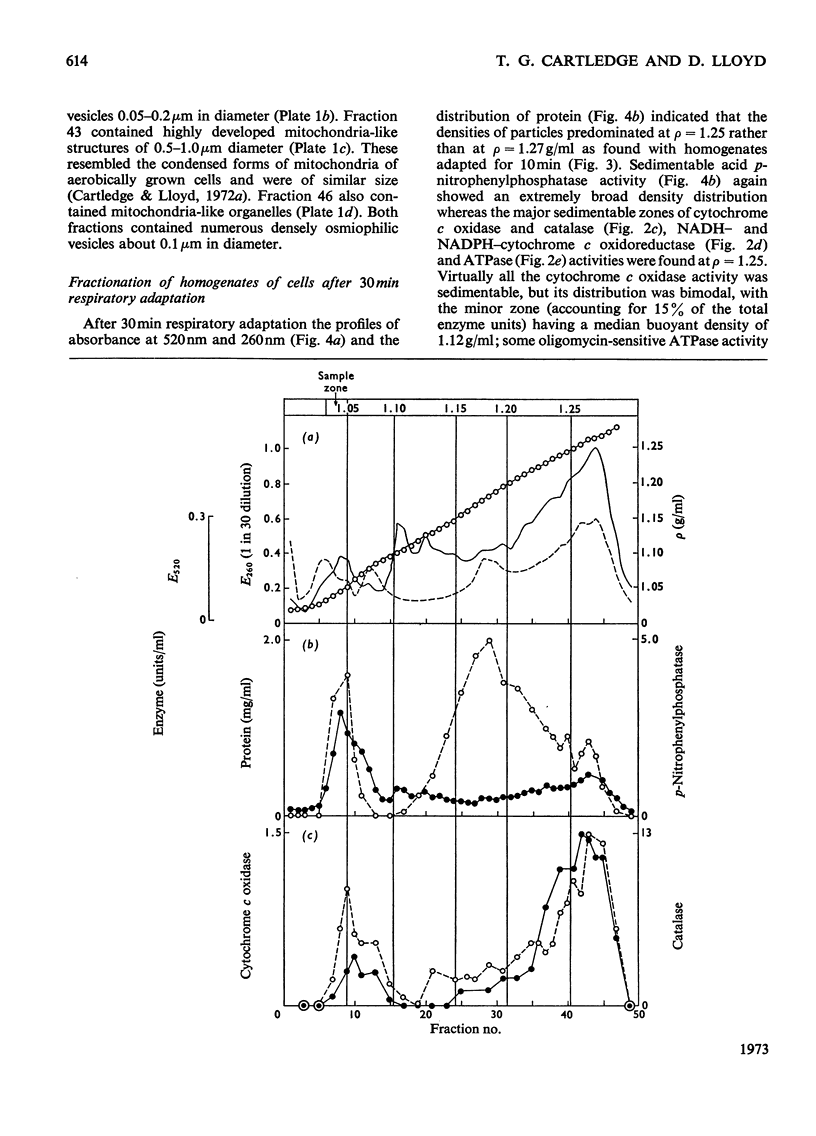
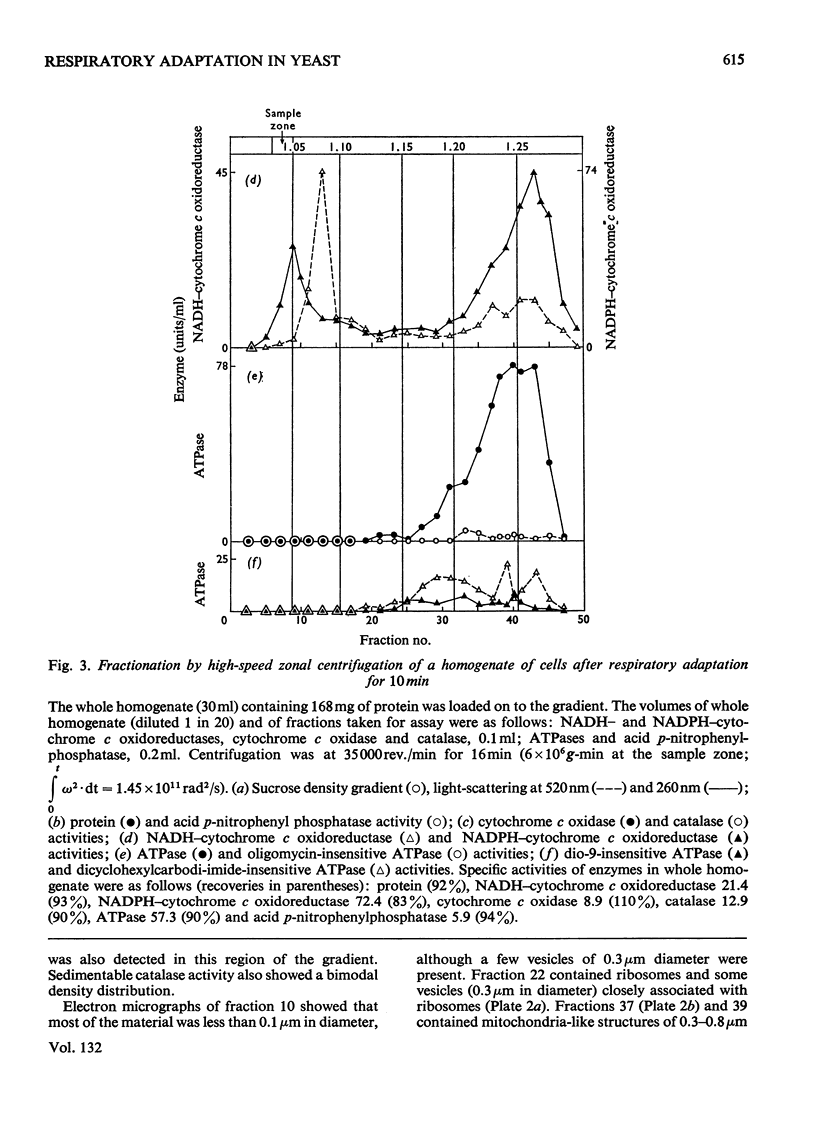
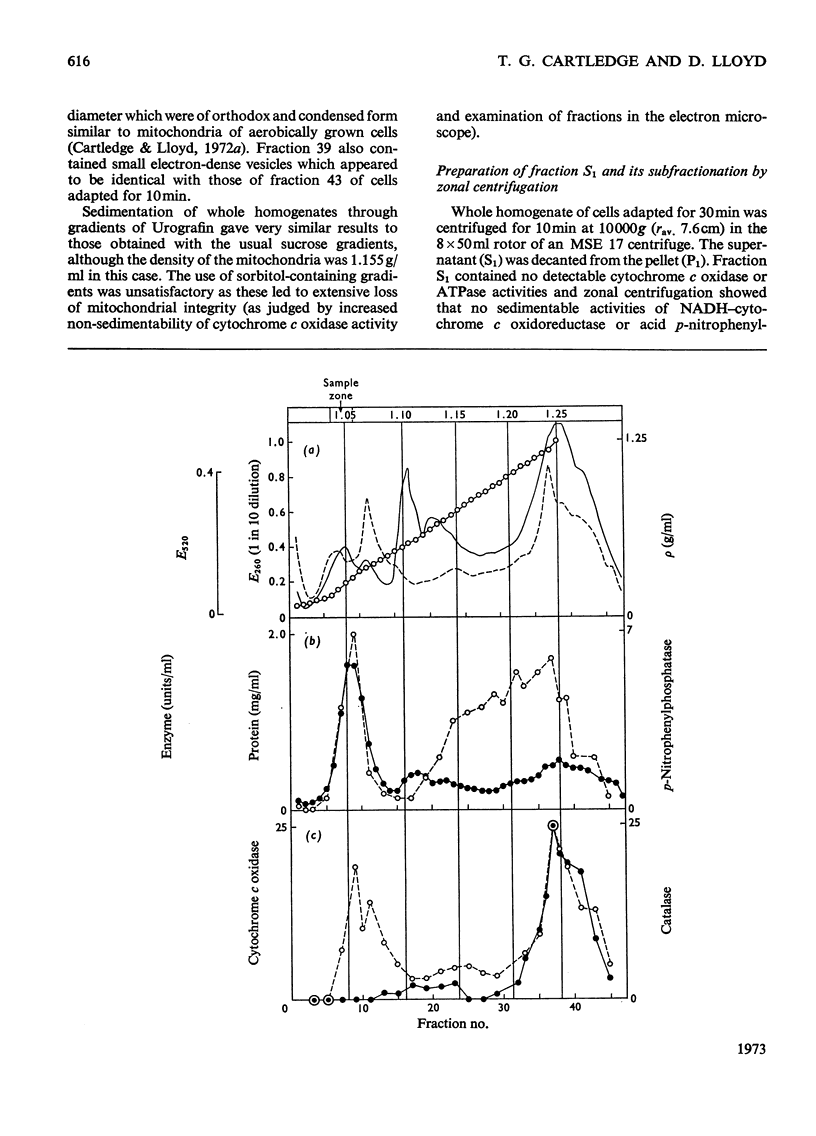
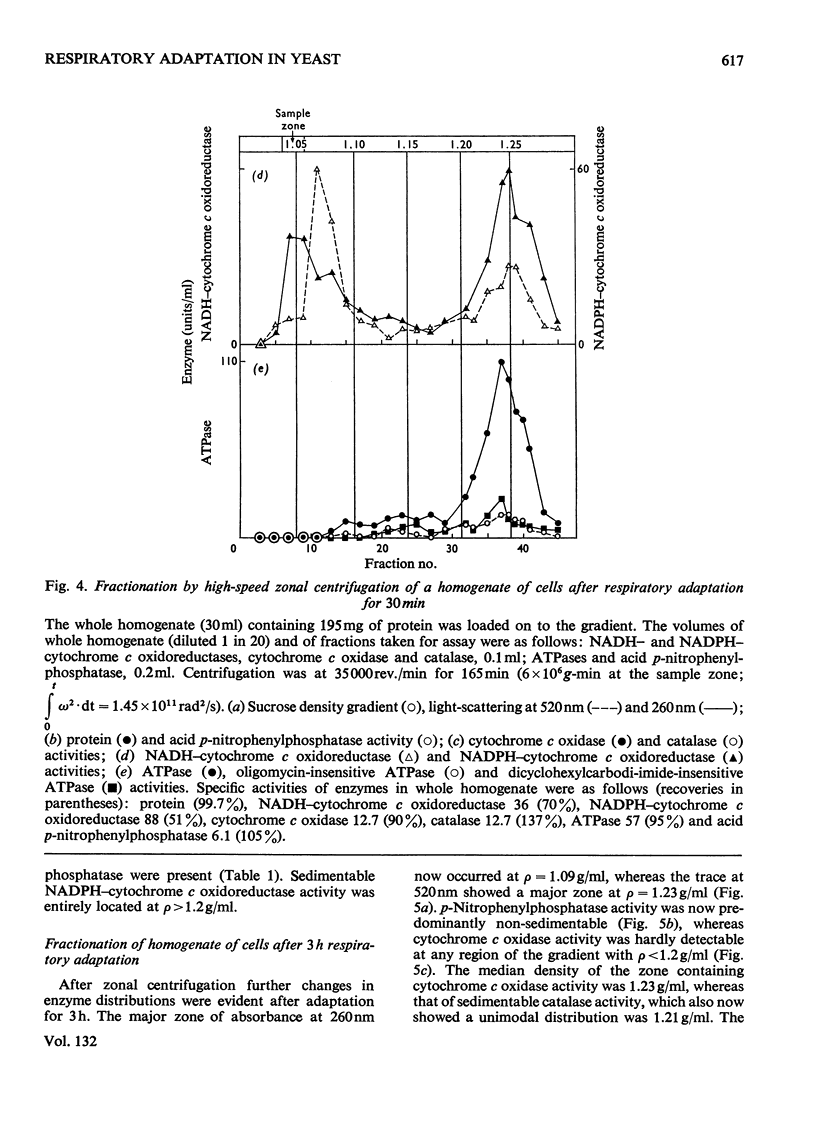
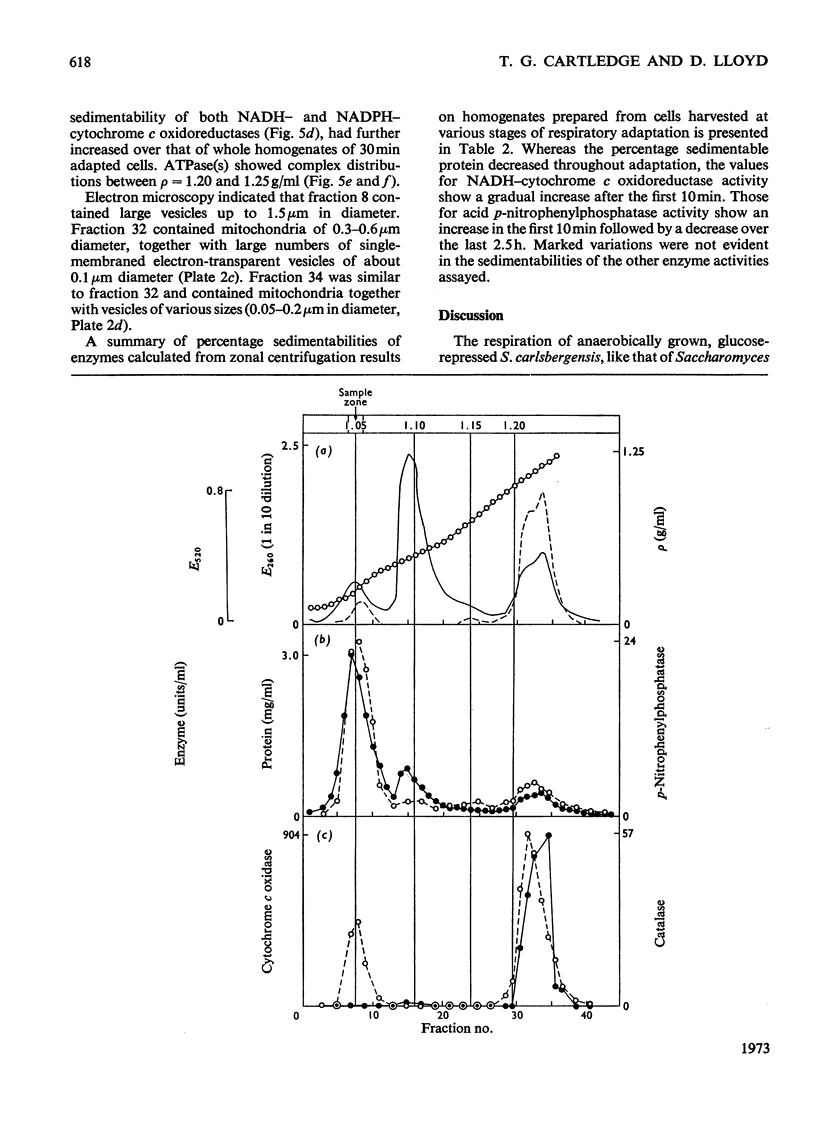
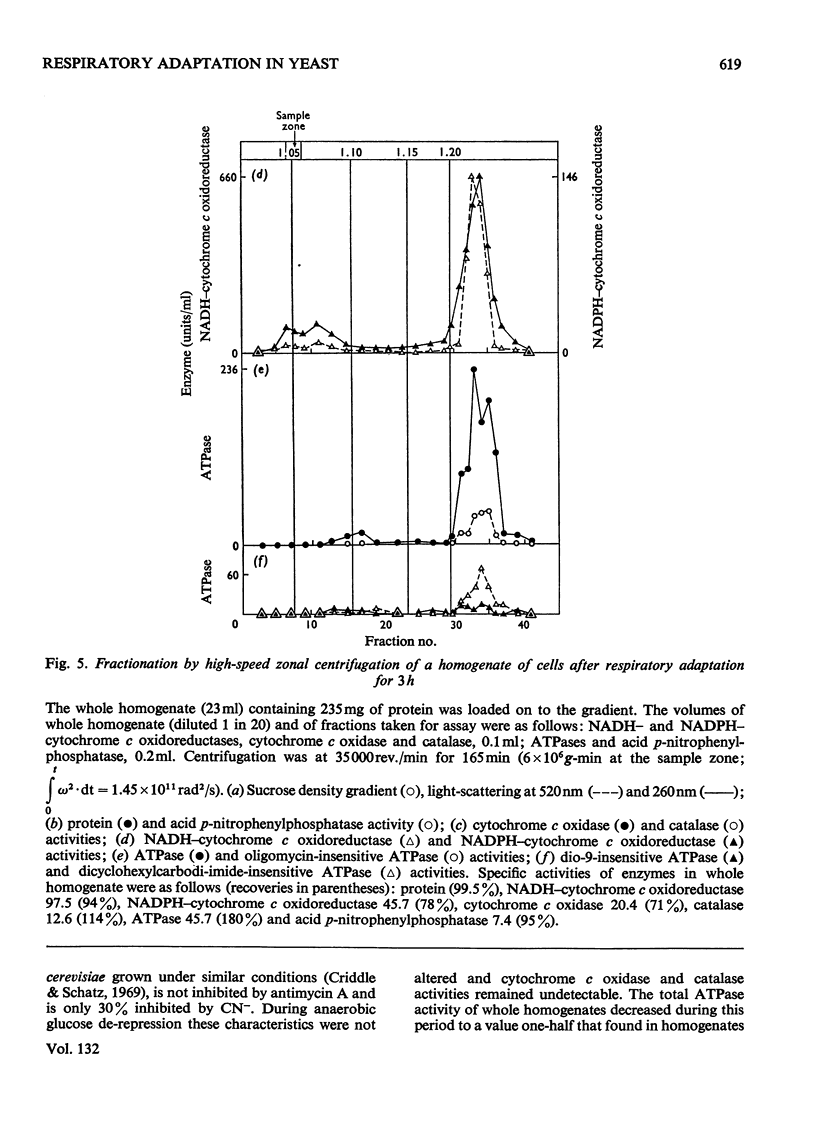
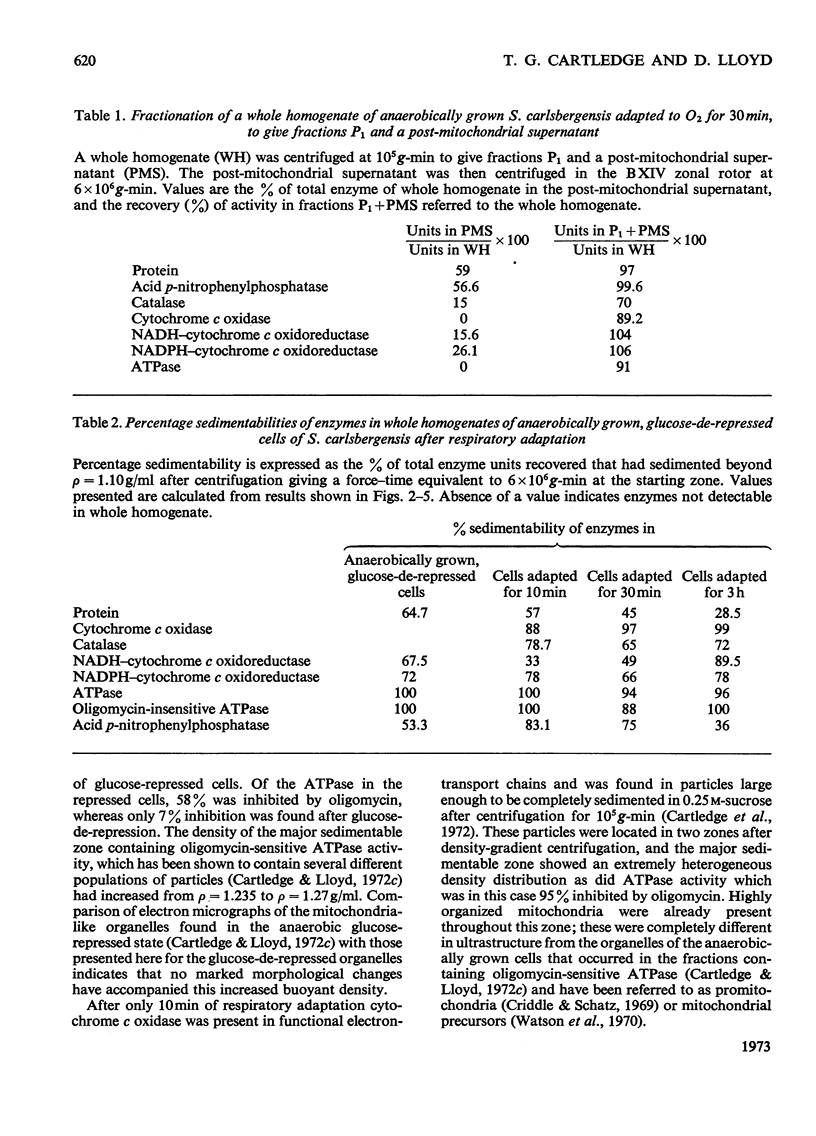
1. During anaerobic glucose de-repression the respiration rate of whole cells of Saccharomyces carlsbergensis remained constant and was insensitive to antimycin A but was inhibited by 30% by KCN. Aeration of cells for 1 h led to increased respiration rate which was inhibited by 80% by antimycin A or KCN. 2. Homogenates were prepared from sphaeroplasts of anaerobically grown, glucose de-repressed cells and the distribution of marker enzymes was investigated after zonal centrifugation on sucrose gradients containing MgCl2. These homogenates contained no detectable cytochrome c oxidase or catalase activity. The complex density distributions of NADH– and NADPH–cytochrome c oxidoreductases and adenosine triphosphatase(s) [ATPase(s)] were very different from those of anaerobically grown, glucose-repressed cells. 3. The specific activity of total ATPase was lowered and sensitivity to oligomycin decreased from 58 to 7% during de-repression. 4. Cytochrome c oxidase and catalase activities were detectable in homogenates of cells after 10min aeration. Zonal centrifugation indicated complex, broad sedimentable distributions of all enzyme activities assayed; the peaks of activity were at 1.27g/ml. 5. Centrifugation of homogenates of cells adapted for 30min and 3 h indicated a shift of density of the major sedimentable peak from 1.25g/ml (30min) to 1.235g/ml (3 h). After 30min adaptation a minor zone of oligomycin-sensitive ATPase and 15% of the total cytochrome c oxidase activities were detected at ρ=1.12g/l; these particles together with those of higher density containing cytochrome c oxidase, ATPase and NADH–cytochrome c oxidoreductase activities were all sedimented at 105g-min. 6. Electron microscopy indicated that the mitochondria-like structures of anaerobically grown, glucose-de-repressed cells were similar to those of repressed cells. After 10min of respiratory adaptation highly organized mitochondria were evident which resembled the condensed forms of mitochondria of aerobically grown, glucose-de-repressed cells. High-density zonal fractions of homogenates of cells after adaptation also contained numerous electron-dense vesicles 0.05–0.2μm in diameter. 7. The possibility that the `promitochondria' of anaerobically grown cells may not be the direct structural precursors of fully functional mitochondria is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avers C. J., Federman M. The occurrence in yeast of cytoplasmic granules which resemble microbodies. J Cell Biol. 1968 May;37(2):555–559. doi: 10.1083/jcb.37.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANTRENNE H., COURTOIS C. Formation de catalase induite par l'oxygène chez la levure. Biochim Biophys Acta. 1954 Jul;14(3):397–400. doi: 10.1016/0006-3002(54)90198-5. [DOI] [PubMed] [Google Scholar]
- Cartledge T. G., Lloyd D., Erecinńska M., Chance B. The development of the respiratory chain of Saccharomyces carlsbergensis during respiratory adaptation. Biochem J. 1972 Dec;130(3):739–747. doi: 10.1042/bj1300739. [DOI] [PMC free article] [PubMed] [Google Scholar]
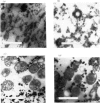
- Cartledge T. G., Lloyd D. Subcellular fractionation by zonal centrifugation of glucose-repressed anaerobically grown Saccharomyces carlsbergensis. Biochem J. 1972 May;127(4):693–703. doi: 10.1042/bj1270693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartledge T. G., Lloyd D. Subcellular fractionation of particles containing acid hydrolases from Saccharomyces carlsbergensis. Biochem J. 1972 Feb;126(3):755–757. doi: 10.1042/bj1260755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. L., Charalampous F. C. Mechanism of induction of cytochrome oxidase in yeast. I. Kinetics of induction and evidence for accumulation of cytoplasmic and mitochondrial precursors. J Biol Chem. 1969 May 25;244(10):2767–2776. [PubMed] [Google Scholar]
- Criddle R. S., Schatz G. Promitochondria of anaerobically grown yeast. I. Isolation and biochemical properties. Biochemistry. 1969 Jan;8(1):322–334. doi: 10.1021/bi00829a045. [DOI] [PubMed] [Google Scholar]
- EPHRUSSI B., SLONIMSKI P. P., PERRODIN G. La synthèse adaptative des cytochromes chez la levure de boulangerie. Biochim Biophys Acta. 1950 Nov;6(2):256–267. doi: 10.1016/0006-3002(50)90098-9. [DOI] [PubMed] [Google Scholar]
- HUGHES D. E. A press for disrupting bacteria and other micro-organisms. Br J Exp Pathol. 1951 Apr;32(2):97–109. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Plattner H., Salpeter M. M., Saltzgaber J., Schatz G. Promitochondria of anaerobically grown yeast. IV. Conversion into respiring mitochondria. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1252–1259. doi: 10.1073/pnas.66.4.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouslin W., Schatz G. Interdependence between promitochondrial and cytoplasmic protein synthesis during respiratory adaptation in baker's yeast. Biochem Biophys Res Commun. 1969 Dec 4;37(6):1002–1007. doi: 10.1016/0006-291x(69)90231-9. [DOI] [PubMed] [Google Scholar]
- Sels A. A., Verhulst A. M. Effects of benzimidazole on the oxygen-induced biosynthesis of hemoproteins in yeast. Eur J Biochem. 1971 Mar 1;19(1):115–123. doi: 10.1111/j.1432-1033.1971.tb01294.x. [DOI] [PubMed] [Google Scholar]
- Somlo M. Induction and repression of mitochondrial ATPase in yeast. Eur J Biochem. 1968 Jul;5(2):276–284. doi: 10.1111/j.1432-1033.1968.tb00368.x. [DOI] [PubMed] [Google Scholar]
- TUSTANOFF E. R., BARTLEY W. THE EFFECT OF GLUCOSE ON THE DEVELOPMENT OF RESPIRATION BY ANAEROBICALLY GROWN YEAST. Can J Biochem. 1964 May;42:651–665. doi: 10.1139/o64-078. [DOI] [PubMed] [Google Scholar]