Abstract
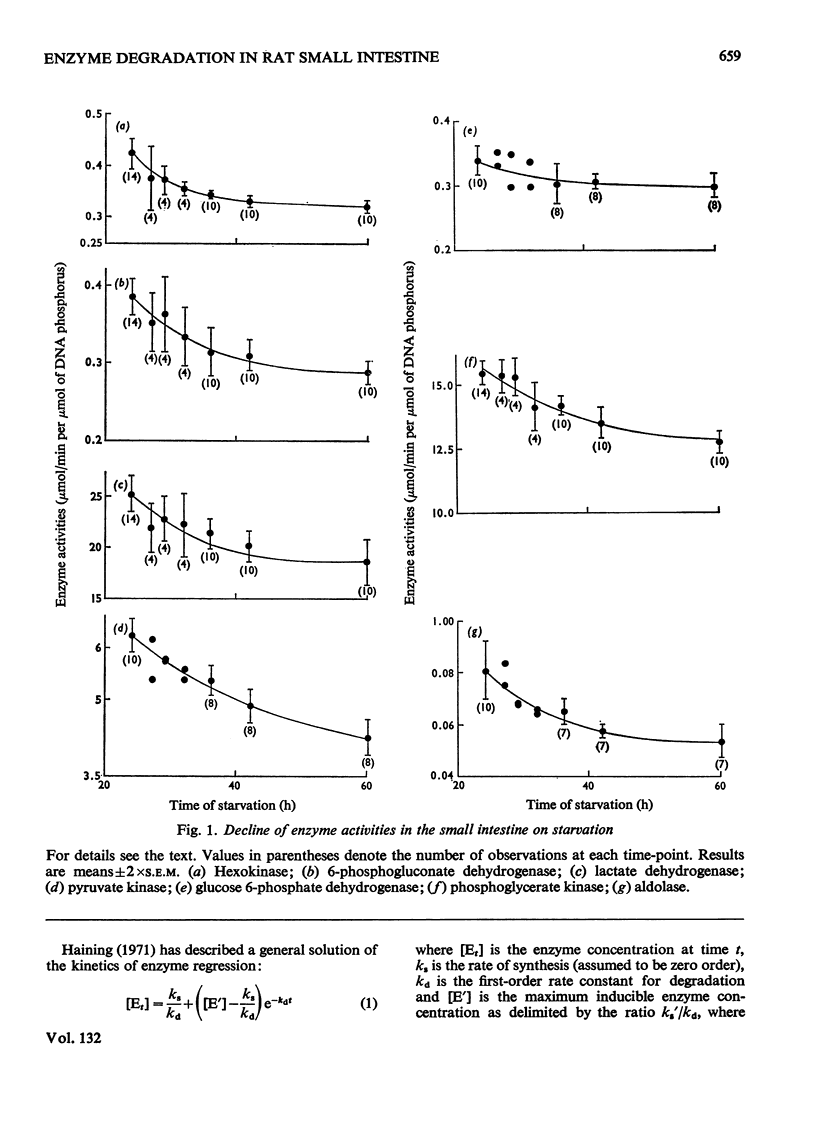
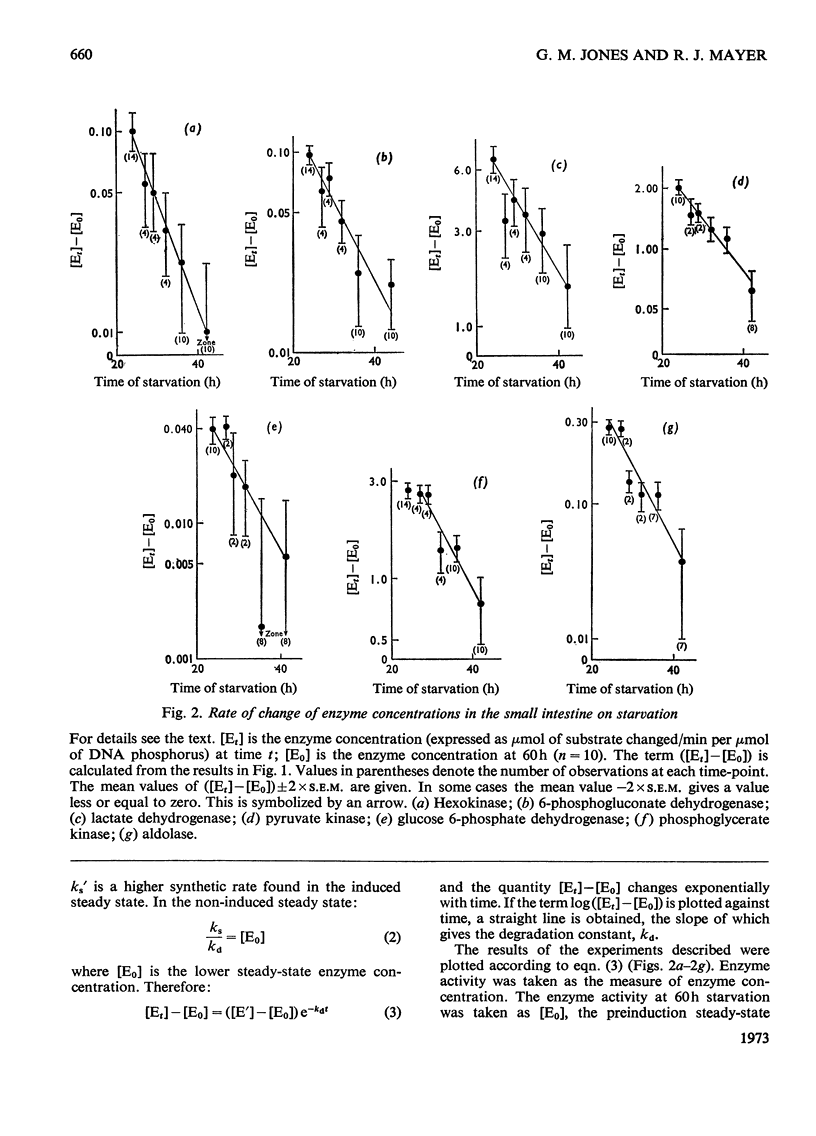
1. The degradation rates and half-lives of hexokinase, 6-phosphogluconate dehydrogenase, lactate dehydrogenase, pyruvate kinase, glucose 6-phosphate dehydrogenase, phosphoglycerate kinase and aldolase were calculated from measurements of the decline in activities of these enzymes in rat small intestine during starvation. 2. The half-lives of the enzymes are: hexokinase, 5.7h; 6-phosphogluconate dehydrogenase, 7.6h; glucose 6-phosphate dehydrogenase, 6.0h; pyruvate kinase, 8.9h; lactate dehydrogenase, 8.7h; phosphoglycerate kinase, 8.7h; aldolase, 5.1h. 3. The significance of the results is discussed with respect to the regulation of enzyme concentrations in response to changes in diet.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. W., Zakim D. The influence of alloxan-diabetes and fasting on glycolytic and gluconeogenic enzyme activities of rat intestinal mucosa and liver. Biochim Biophys Acta. 1970 Feb 24;201(2):236–241. doi: 10.1016/0304-4165(70)90297-7. [DOI] [PubMed] [Google Scholar]
- BERTALANFFY F. D. Mitotic rates and renewal times of the digestive tract epithelia in the rat. Acta Anat (Basel) 1960;40:130–148. doi: 10.1159/000141580. [DOI] [PubMed] [Google Scholar]
- Creamer B. The turnover of the epithelium of the small intestine. Br Med Bull. 1967 Sep;23(3):226–230. doi: 10.1093/oxfordjournals.bmb.a070561. [DOI] [PubMed] [Google Scholar]
- Fritz P. J., Vesell E. S., White E. L., Pruitt K. M. The roles of synthesis and degradation in determining tissue concentrations of lactate dehydrogenase-5. Proc Natl Acad Sci U S A. 1969 Feb;62(2):558–565. doi: 10.1073/pnas.62.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haining J. L. On the kinetics of liver enzyme regression following induction. Arch Biochem Biophys. 1971 May;144(1):204–208. doi: 10.1016/0003-9861(71)90469-3. [DOI] [PubMed] [Google Scholar]
- Hübscher G., West G. R., Brindley D. N. Studies on the fractionation of mucosal homogenates from the small intestine. Biochem J. 1965 Dec;97(3):629–642. doi: 10.1042/bj0970629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. P., Alpers D. H., Gerber J. E., Isselbacher K. J. The turnover of disaccharidases and brush border proteins in rat intestine. Biochim Biophys Acta. 1971 Feb 23;230(2):194–203. doi: 10.1016/0304-4165(71)90204-2. [DOI] [PubMed] [Google Scholar]
- Kuehl L., Sumsion E. N. Turnover of several glycolytic enzymes in rat liver. J Biol Chem. 1970 Dec 25;245(24):6616–6623. [PubMed] [Google Scholar]
- Rudack D., Chisholm E. M., Holten D. Rat liver glucose 6-phosphate dehydrogenase. Regulation by carbohydrate diet and insulin. J Biol Chem. 1971 Mar 10;246(5):1249–1254. [PubMed] [Google Scholar]
- Rudack D., Gozukara E. M., Chisholm E. M., Holten D. The effect of dietary carbohydrate and fat on the synthesis of rat liver 6-phosphogluconate dehydrogenase. Biochim Biophys Acta. 1971 Nov 12;252(2):305–313. doi: 10.1016/0304-4165(71)90011-0. [DOI] [PubMed] [Google Scholar]
- Srivastava L. M., Hübscher G. Glucose metabolism in the mucosa of the small intestine. Enzymes of the pentose phosphate pathway. Biochem J. 1966 Oct;101(1):48–55. doi: 10.1042/bj1010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava L. M., Shakespeare P., Hübscher G. Glucose metabolism in the mucosa of the small intestine. A study of hexokinase activity. Biochem J. 1968 Aug;109(1):35–42. doi: 10.1042/bj1090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szepesi B., Freedland R. A. Time course of enzyme adaptation. 3. Periodic and nonperiodic responses of rat liver enzyme activities to diets in meal-fed rats. Can J Biochem. 1971 Jan;49(1):108–118. doi: 10.1139/o71-016. [DOI] [PubMed] [Google Scholar]




