Abstract
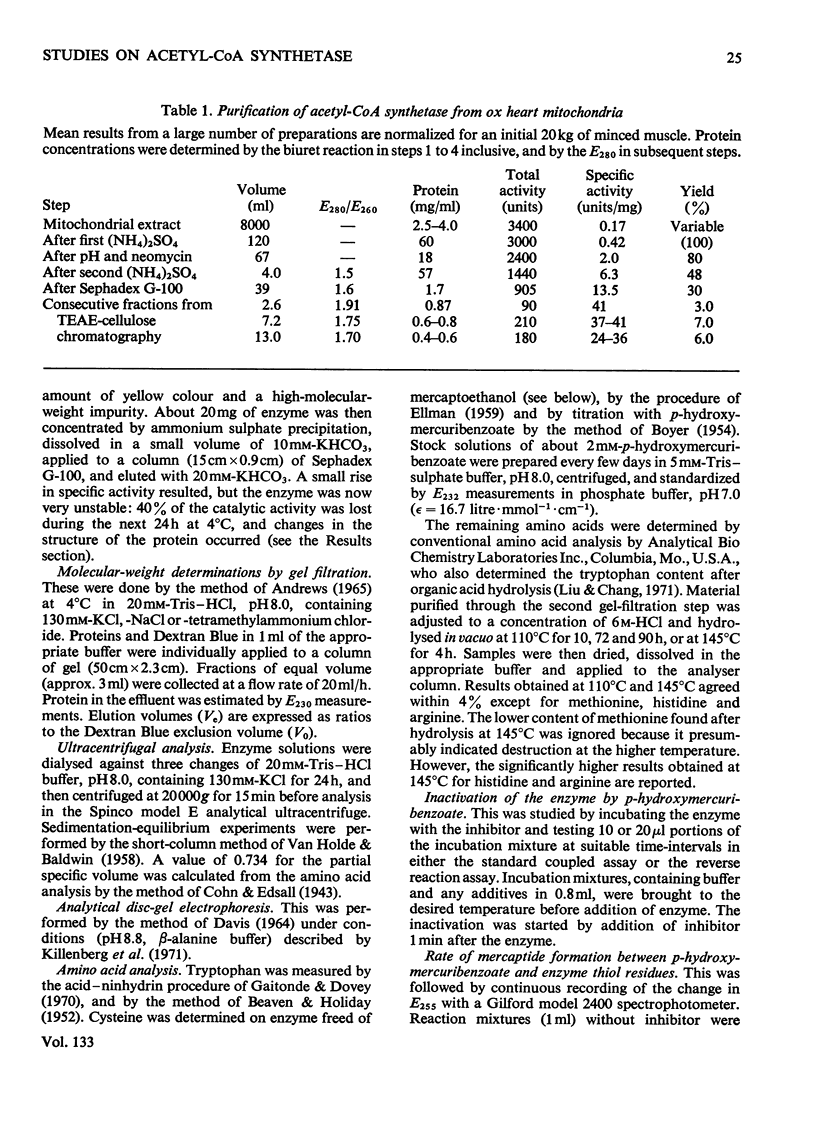
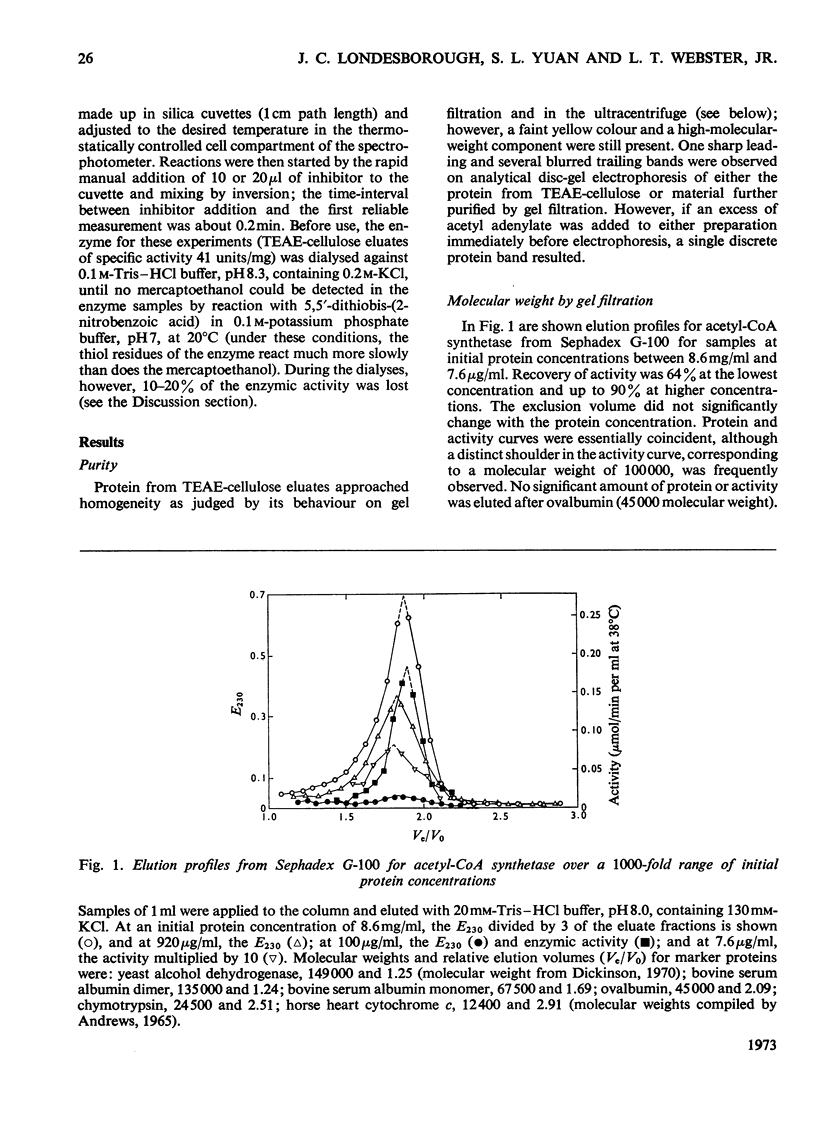
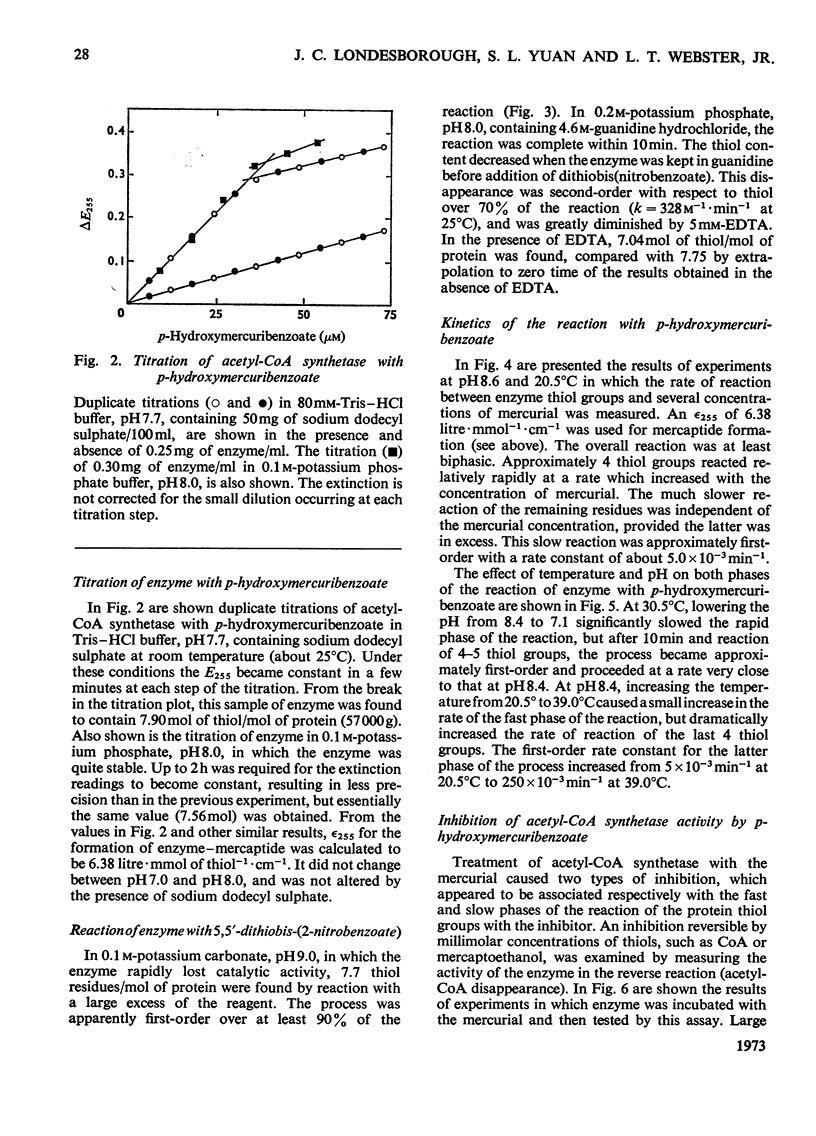
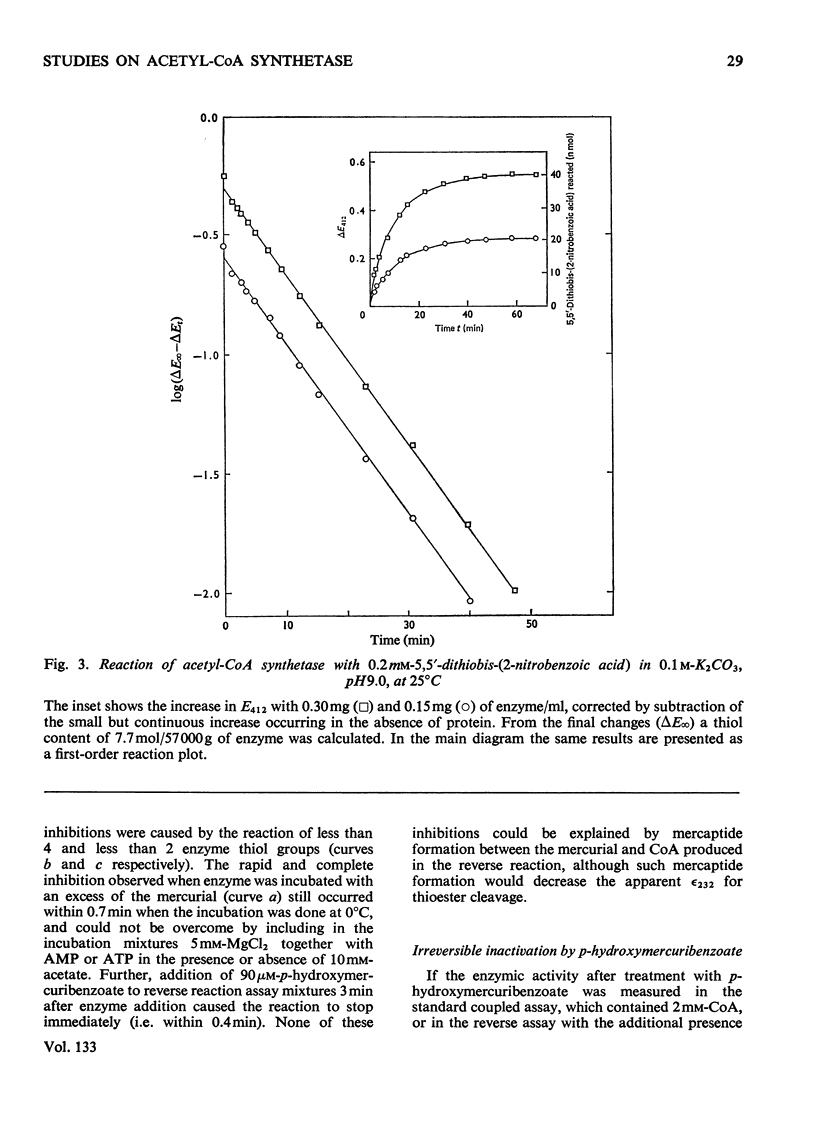
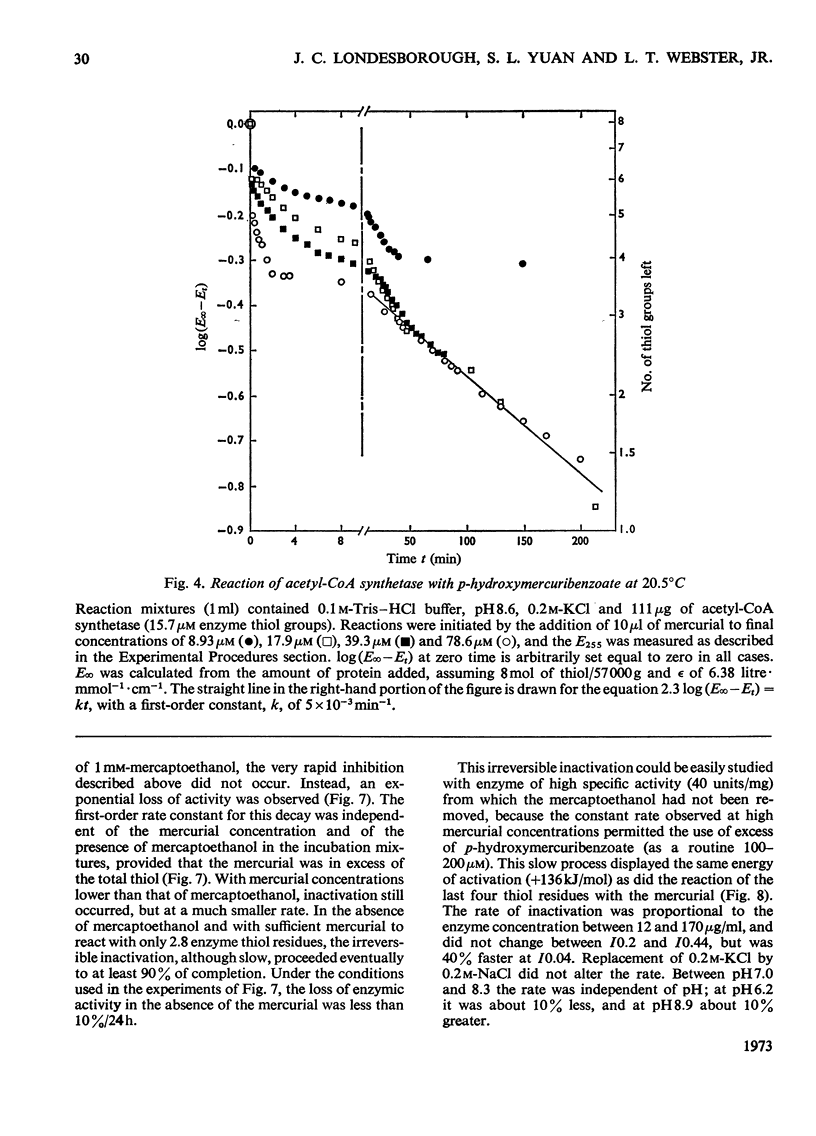
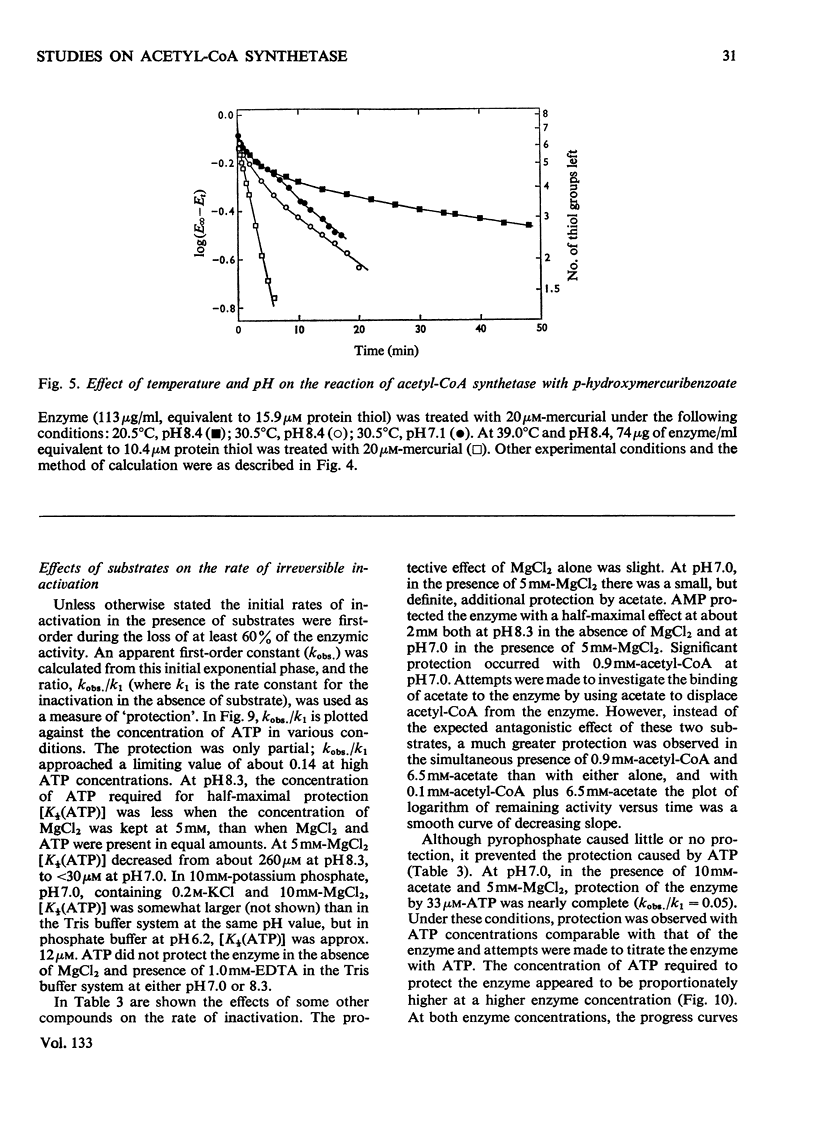
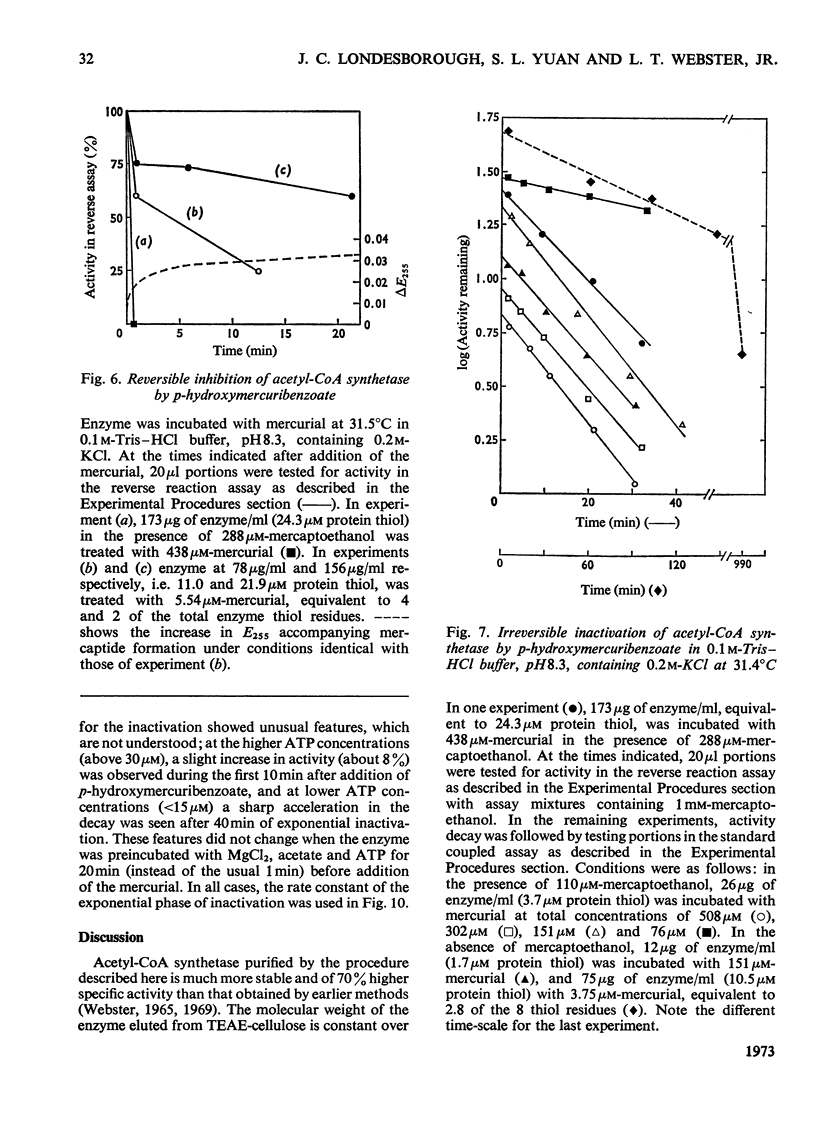
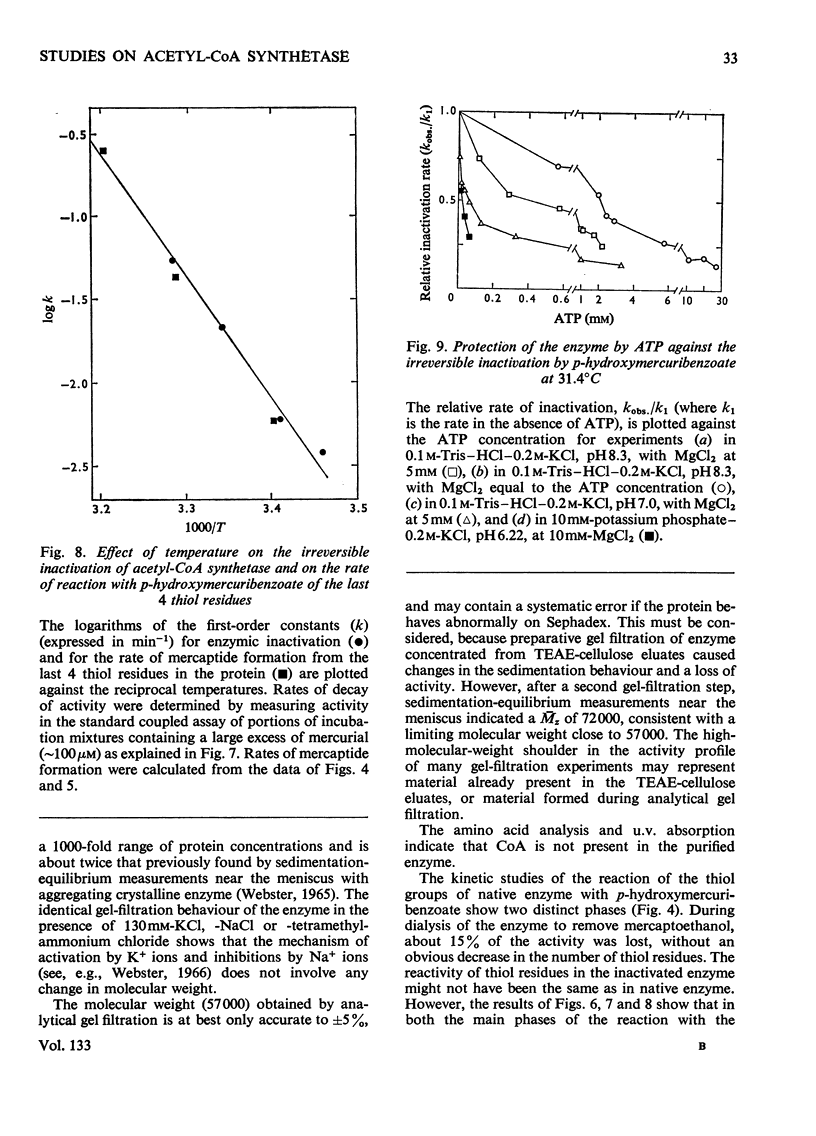
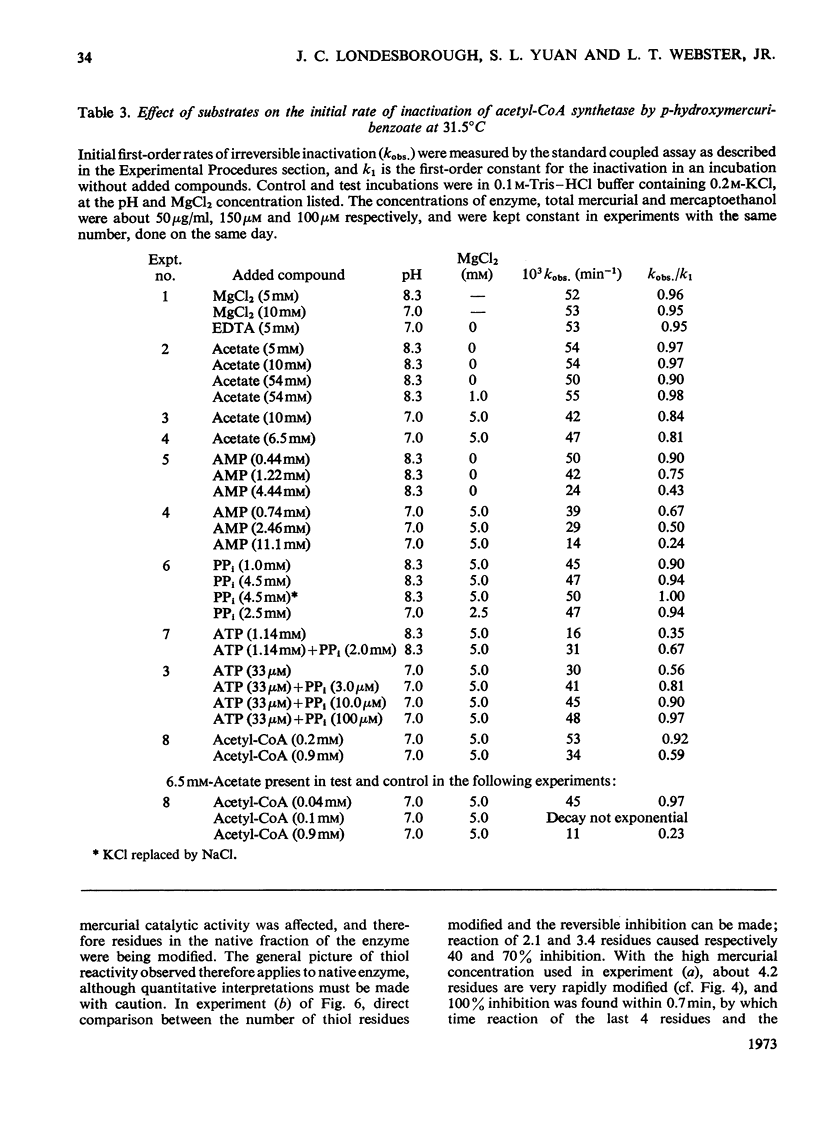
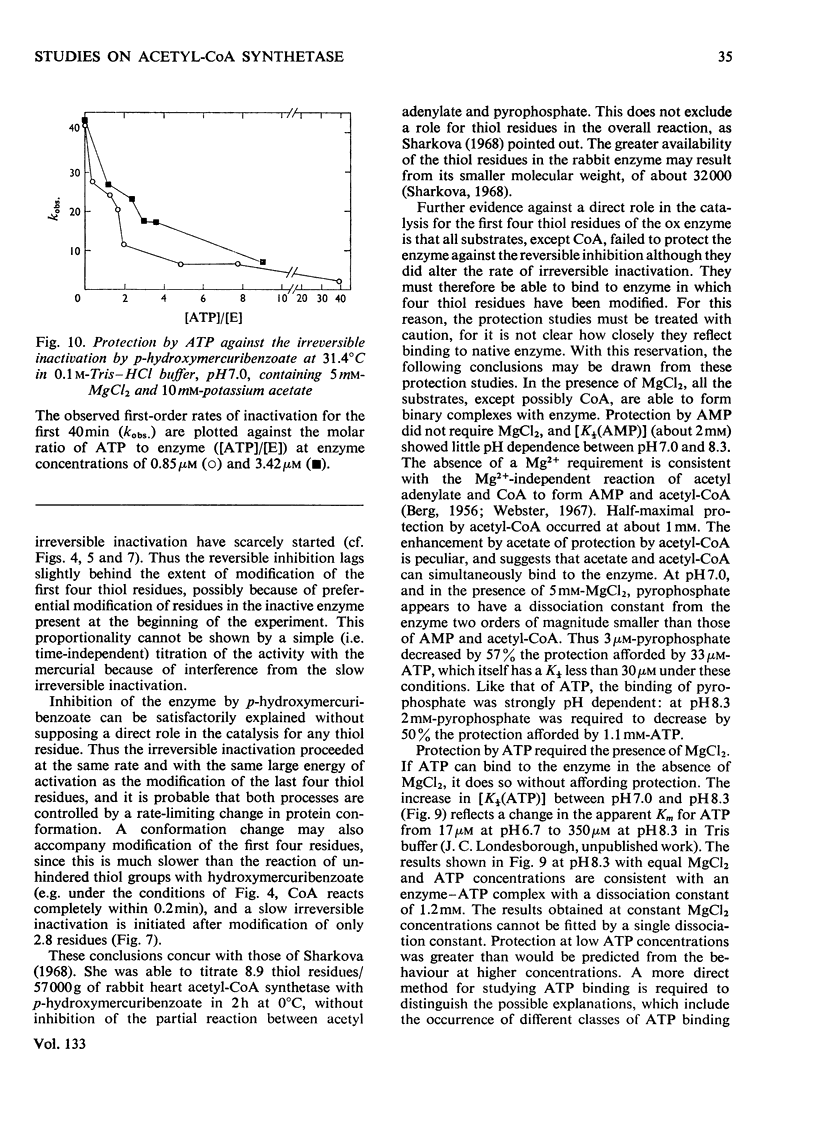
1. A constant molecular weight of 57000 was obtained by gel filtration of highly purified acetyl-CoA synthetase over a 1000-fold range of enzyme concentrations. The amino acid analysis is reported. 2. With native enzyme at 20°C the relatively rapid reaction of four thiol residues with p-hydroxymercuribenzoate caused an immediate inhibition reversible by either CoA or mercaptoethanol. Other substrates did not protect against this rapid inhibition. 3. The much slower reaction of the remaining four thiol residues was independent of the concentration of the mercurial, first-order with respect to enzyme, and had a large energy of activation (+136kJ/mol), suggesting that a conformation change in the protein was rate-limiting. This slow phase of the reaction was accompanied by an irreversible inactivation of the enzyme. 4. The effects of substrates on this irreversible inactivation at pH7.0 in 5 mm-MgCl2 indicated strong binding of ATP and pyrophosphate by the enzyme (concentrations for half-maximal effects, K½, were <30μm and <10μm respectively) and weaker binding of acetyl-CoA (K½ about 1 mm), AMP (K½ about 2mm) and acetate. In the presence of acetate, MgCl2 and p-hydroxymercuribenzoate, titration of the enzyme with ATP revealed at least two ATP binding sites/mol. 5. The experiments suggest that reaction of the thiol residues with mercurial causes loss of enzymic activity by altering the structure of the enzyme, rather than that the thiol residues play a direct role in the catalysis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEAVEN G. H., HOLIDAY E. R. Ultraviolet absorption spectra of proteins and amino acids. Adv Protein Chem. 1952;7:319–386. doi: 10.1016/s0065-3233(08)60022-4. [DOI] [PubMed] [Google Scholar]
- BERG P. Acyl adenylates; an enzymatic mechanism of acetate activation. J Biol Chem. 1956 Oct;222(2):991–1013. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dickinson F. M. The binding of dihydronicotinamide--adenine dinucleotide and pyridine-3-aldehyde--adenine dinucleotide by yeast alcohol dehydrogenase. Biochem J. 1970 Dec;120(4):821–830. doi: 10.1042/bj1200821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Gaitonde M. K., Dovey T. A rapid and direct method for the quantitative determination of tryptophan in the intact protein. Biochem J. 1970 May;117(5):907–911. doi: 10.1042/bj1170907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELE P. The acetate activating enzyme of beef heart. J Biol Chem. 1954 Feb;206(2):671–676. [PubMed] [Google Scholar]
- Killenberg P. G., Davidson E. D., Webster L. T., Jr Evidence for a medium-chain fatty acid: coenzyme A ligase (adenosine monophosphate) that activates salicylate. Mol Pharmacol. 1971 May;7(3):260–268. [PubMed] [Google Scholar]
- Lee R., McElroy W. D. Role and reactivity of sulfhydryl groups in firefly luciferase. Biochemistry. 1969 Jan;8(1):130–136. doi: 10.1021/bi00829a018. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Chang Y. H. Hydrolysis of proteins with p-toluenesulfonic acid. Determination of tryptophan. J Biol Chem. 1971 May 10;246(9):2842–2848. [PubMed] [Google Scholar]
- Loftfield R. B., Eigner E. A. Mechanism of action of amino acid transfer ribonucleic acid ligases. J Biol Chem. 1969 Apr 10;244(7):1746–1754. [PubMed] [Google Scholar]
- Sharkova E. V. Vydelenie, ochistka i svoistva atsetil-KoA-sintetazy iz serdtsa krolika. Biokhimiia. 1968 Jul-Aug;33(4):792–799. [PubMed] [Google Scholar]
- Webster L. T., Jr Studies of the acetyl coenzyme A synthetase reaction. II. Crystalline acetyl coenzyme A synthetase. J Biol Chem. 1965 Nov;240(11):4158–4163. [PubMed] [Google Scholar]
- Webster L. T., Jr Studies of the acetyl coenzyme A synthetase reaction. IV. The requirement for monovalent cations. J Biol Chem. 1966 Dec 10;241(23):5504–5510. [PubMed] [Google Scholar]
- Webster L. T., Jr Studies of the acetyl coenzyme A synthetase reaction. V. The requirement for monovalent and divalent cations in partial reactions involving enzyme-bound acetyl adenylate. J Biol Chem. 1967 Mar 25;242(6):1232–1240. [PubMed] [Google Scholar]


