Abstract
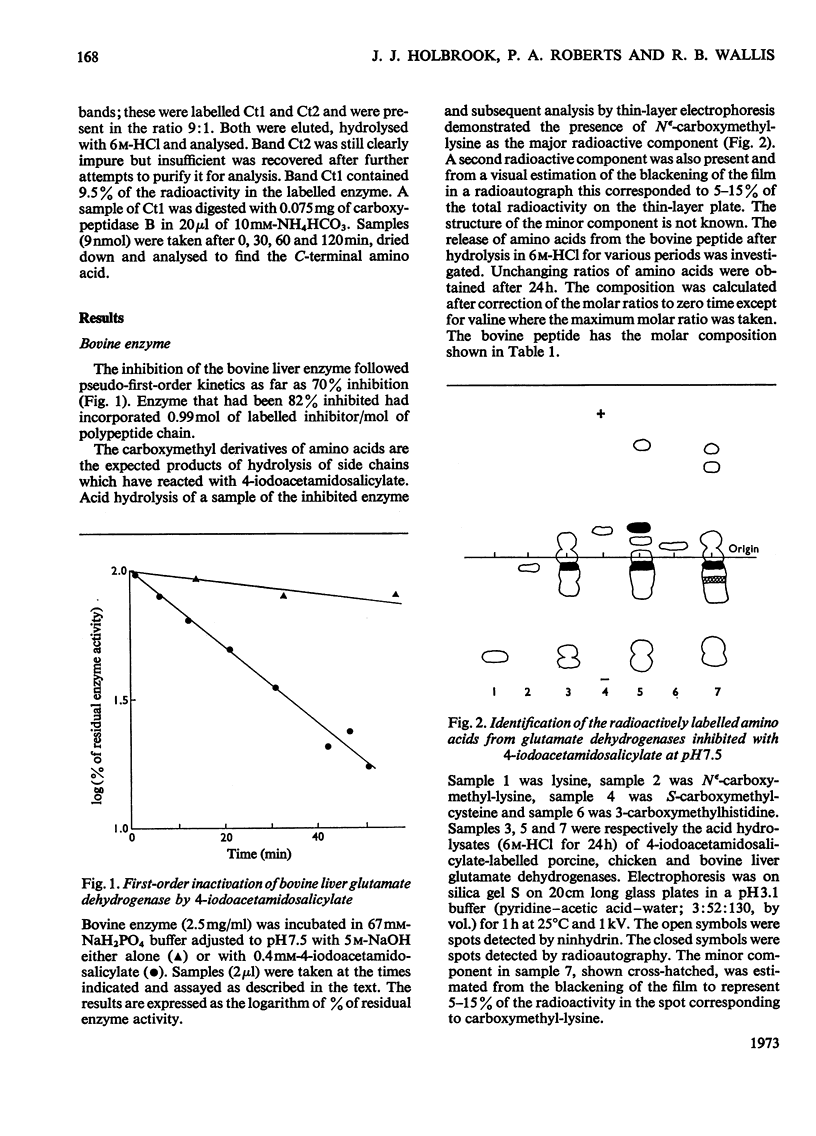
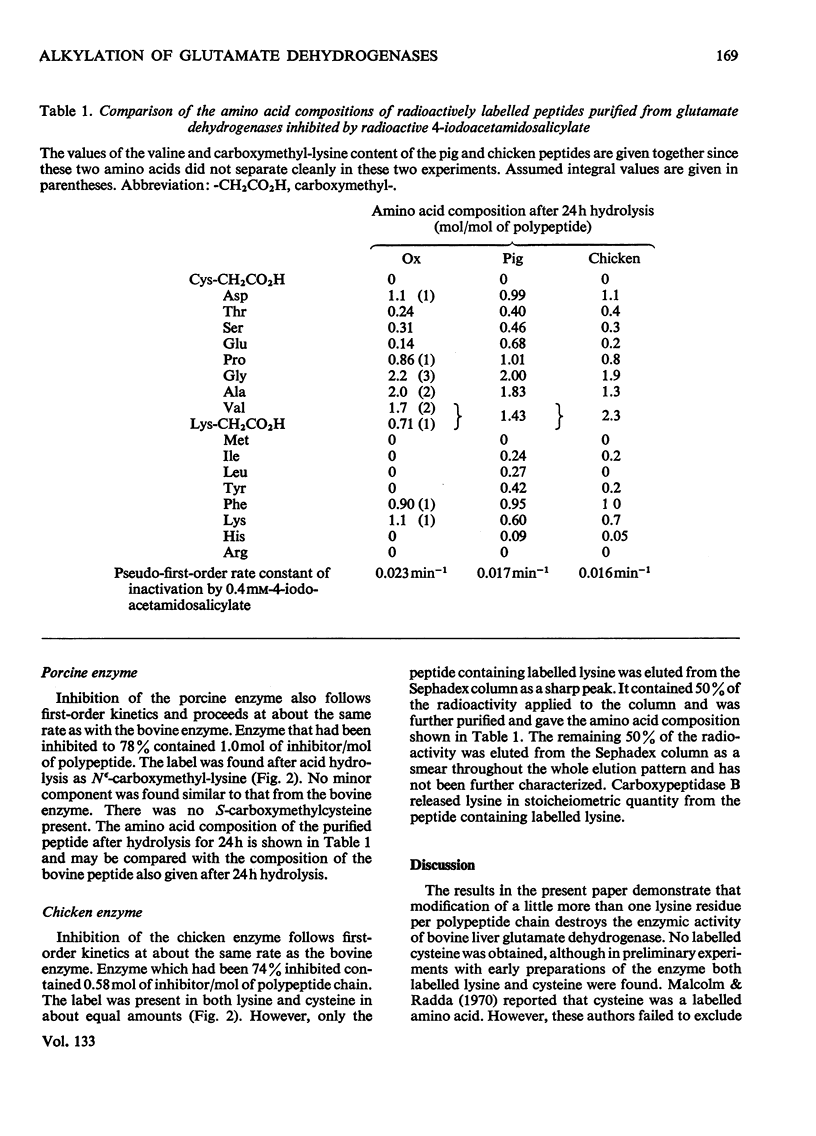
1. Bovine, porcine and chicken liver glutamate dehydrogenases were irreversibly inhibited by a tenfold excess of radioactive 4-iodoacetamidosalicylic acid at pH7.5. 2. Inhibition was accompanied by the covalent incorporation of 1.1 mol of labelled inhibitor/mol of polypeptide chain. Acid hydrolysis yielded Nε-carboxymethyl-lysine as the sole labelled amino acid. No labelled S-carboxymethylcysteine was recovered from the bovine or porcine enzymes. 3. The labelled bovine enzyme was hydrolysed with trypsin. The radioactivity was found at lysine-126 in a peptide comprising residues 119–130 of the sequence. 4. The amino acid compositions of the tryptic peptides containing labelled lysine from the porcine and chicken enzymes were similar to that of the bovine peptide.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P. THE AMINO ACID SEQUENCE OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:349–378. doi: 10.1042/bj0890349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRESTFIELD A. M., STEIN W. H., MOORE S. Alkylation and identification of the histidine residues at the active site of ribonuclease. J Biol Chem. 1963 Jul;238:2413–2419. [PubMed] [Google Scholar]
- Coffee C. J., Bradshaw R. A., Goldin B. R., Frieden C. Identification of the sites of modification of bovine liver glutamate dehydrogenase reacted with trinitrobenzenesulfonate. Biochemistry. 1971 Sep 14;10(19):3516–3526. doi: 10.1021/bi00795a005. [DOI] [PubMed] [Google Scholar]
- Colman R. F., Frieden C. On the role of amino groups in the structure and function of glutamate dehydrogenase. I. Effect of acetylation on catalytic and regulatory properties. J Biol Chem. 1966 Aug 25;241(16):3652–3660. [PubMed] [Google Scholar]
- Corman L., Prescott L. M., Kaplan N. O. Purification and kinetic characteristics of dogfish liver glutamate dehydrogenase. J Biol Chem. 1967 Apr 10;242(7):1383–1390. [PubMed] [Google Scholar]
- GUNDLACH H. G., STEIN W. H., MOORE S. The nature of the amino acid residues involved in the inactivation of ribonuclease by iodoacetate. J Biol Chem. 1959 Jul;234(7):1754–1760. [PubMed] [Google Scholar]
- Goldin B. R., Frieden C. The effect of pyridoxal phosphate modification on the catalytic and regulatory properties of bovine liver glutamate dehydrogenase. J Biol Chem. 1972 Apr 10;247(7):2139–2144. [PubMed] [Google Scholar]
- Holbrook J. J., Jeckel R. A peptide containing a reactive lysyl group from ox liver glutamate dehydrogenase. Biochem J. 1969 Mar;111(5):689–694. doi: 10.1042/bj1110689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ A. M., DREYER W. J., ANFINSEN C. B. Peptide separation by two-dimensional chromatography and electrophoresis. J Biol Chem. 1959 Nov;234:2897–2900. [PubMed] [Google Scholar]
- Malcolm A. D., Radda G. K. The reaction of glutamate dehydrogenase with 4-iodoacetamido salicylic acid. Eur J Biochem. 1970 Sep;15(3):555–561. doi: 10.1111/j.1432-1033.1970.tb01040.x. [DOI] [PubMed] [Google Scholar]
- Moon K., Piszkiewicz D., Smith E. L. Glutamate dehydrogenase: amino-acid sequence of the bovine enzyme and comparison with that from chicken liver. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1380–1383. doi: 10.1073/pnas.69.6.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piszkiewicz D., Landon M., Smith E. L. Bovine liver flutamate dehydrogenase. Sequence of a hexadecapeptide containing a lysyl residue reactive with pyridoxal 5'-phosphate. J Biol Chem. 1970 May 25;245(10):2622–2626. [PubMed] [Google Scholar]
- SUND H., AKESON A. DIE AMINOSAEUREZUSAMMENSETZUNG DER GLUTAMINSAEUREDEHYDROGENASE AUS RINDERLEBER. Biochem Z. 1964 Sep 28;340:421–435. [PubMed] [Google Scholar]


