Abstract
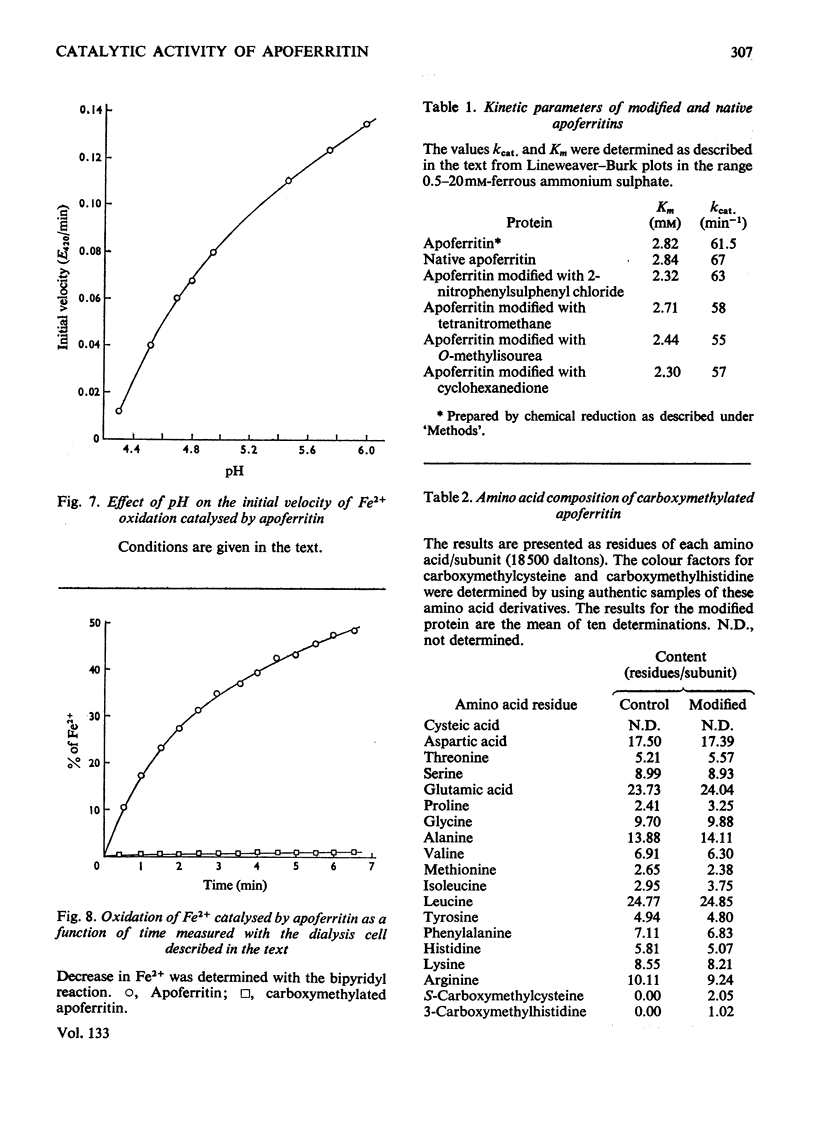
1. Horse spleen apoferritin catalyses the oxidation of Fe2+ to Fe3+ with molecular O2 as electron acceptor under conditions where a number of other proteins have no such effect. The product is similar to ferritin by a number of criteria. 2. The progress curve is hyperbolic and the increase in initial velocity is linear with increasing apoferritin concentration. With respect to Fe2+ the reaction follows Michaelis–Menten kinetics. The pH-dependence of the reaction was determined between pH4.3 and 6.0. 3. Modification of both tryptophan residues/apoferritin subunit with 2-nitrophenylsulphenyl chloride does not affect either kcat. or Km for the oxidation. Neither does the guanidination of seven out of nine lysine residues/subunit, the modification of nine out of ten arginine residues/subunit with cyclohexanedione, or the nitration of one out of five tyrosine residues/subunit with tetranitromethane. 4. The carboxymethylation of two out of three cysteine residues/subunit and of one out of six histidine residues/subunit can be achieved with iodoacetic acid. This carboxymethylated apoferritin is completely inactive in Fe2+ oxidation. 5. Apoferritin does not take up Fe3+. It appears from these results that Fe2+ is the form in which iron is taken up by ferritin in a reaction where the protein acts as an enzyme which traps the product in the interior of the protein shell.
Full text
PDF



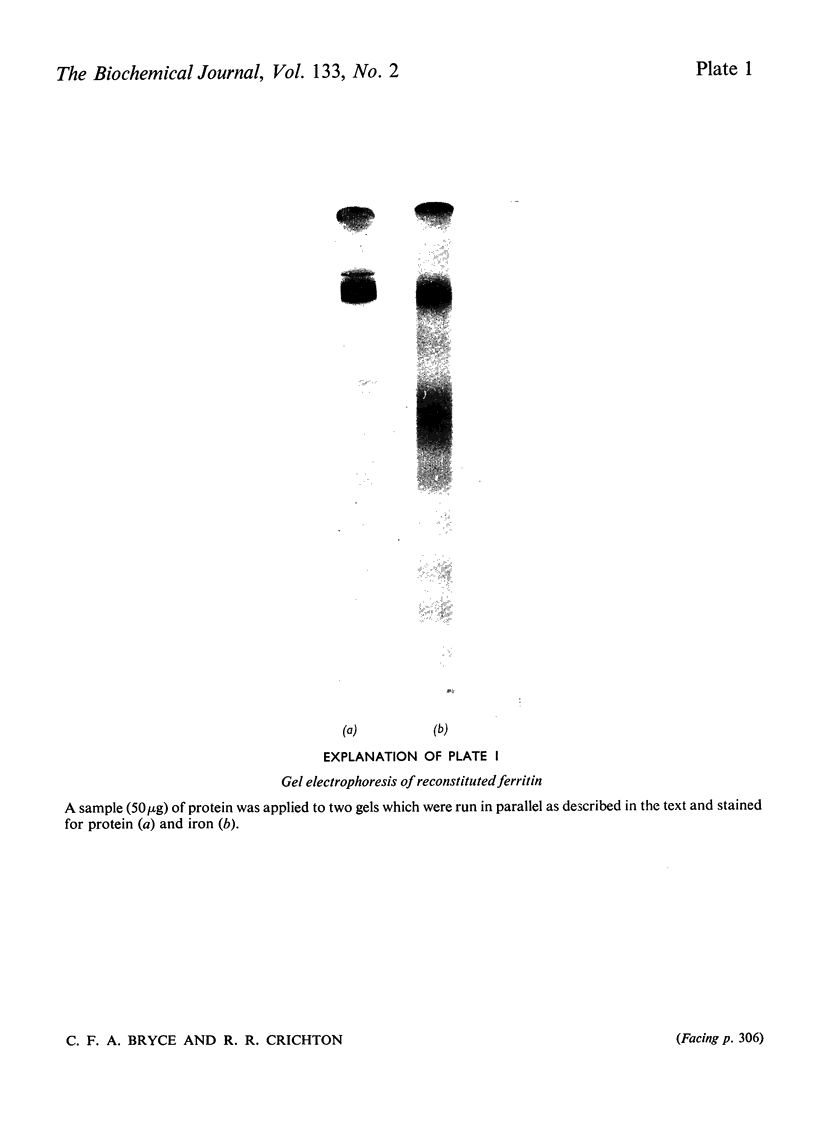



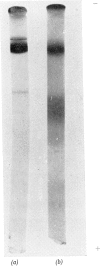
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boccù E., Veronese F. M., Fontana A., Benassi C. A. Sulfenyl halides as modifying reagents for polypeptides and proteins. Quantitative evaluation of tryptophan and cystei residues in proteins. Eur J Biochem. 1970 Mar 1;13(1):188–192. doi: 10.1111/j.1432-1033.1970.tb00917.x. [DOI] [PubMed] [Google Scholar]
- Bryce C. F., Crichton R. R. The subunit structure of horse spleen apoferritin. I. The molecular weight of the subunit. J Biol Chem. 1971 Jul 10;246(13):4198–4205. [PubMed] [Google Scholar]
- CANFIELD R. E., ANFINSEN C. B. CHROMATOGRAPHY OF PEPSIN AND CHYMOTRYPSIN DIGESTS OF EGG WHITE LYSOZYME ON PHOSPHOCELLULOSE. J Biol Chem. 1963 Aug;238:2684–2690. [PubMed] [Google Scholar]
- Coleman C. B., Matrone G. In vivo effect of zinc on iron-induced ferritin synthesis in rat liver. Biochim Biophys Acta. 1969 Feb 18;177(1):106–112. doi: 10.1016/0304-4165(69)90069-5. [DOI] [PubMed] [Google Scholar]
- Crichton R. R., Bryce C. F.A. Molecular weight estimation of apoferritin sub-units. FEBS Lett. 1970 Jan 26;6(2):121–124. doi: 10.1016/0014-5793(70)80017-5. [DOI] [PubMed] [Google Scholar]
- Crichton R. R., Bryce C. F. Subunit interactions in horse spleen apoferritin. Dissociation by extremes of pH. Biochem J. 1973 Jun;133(2):289–299. doi: 10.1042/bj1330289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton R. R., Eason R., Barclay A., Bryce C. F. The subunit structure of horse spleen apoferritin; the molecular weight of the oligomer and its stability to dissociation by dilution. Biochem J. 1973 Apr;131(4):855–857. doi: 10.1042/bj1310855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton R. R., Millar J. A., Cumming R. L., Bryce C. F. The organ-specificity of ferritin in human and horse liver and spleen. Biochem J. 1973 Jan;131(1):51–59. doi: 10.1042/bj1310051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton R. R. The subunit structure of apoferritin and other eicosamers. Biochem J. 1972 Feb;126(3):761–764. doi: 10.1042/bj1260761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale J. W., Haggis G. H., Harrison P. M. Biosynthesis of ferritin molecules. Nature. 1968 Sep 7;219(5158):1045–1046. doi: 10.1038/2191045a0. [DOI] [PubMed] [Google Scholar]
- Fischbach F. A., Anderegg J. W. An x-ray scattering study of ferritin and apoferritin. J Mol Biol. 1965 Dec;14(2):458–473. doi: 10.1016/s0022-2836(65)80196-6. [DOI] [PubMed] [Google Scholar]
- Harrison P. M., Fischbach F. A., Hoy T. G., Haggis G. H. Ferric oxyhydroxide core of ferritin. Nature. 1967 Dec 23;216(5121):1188–1190. doi: 10.1038/2161188a0. [DOI] [PubMed] [Google Scholar]
- LOEWUS M. W., FINEBERG R. A. The incorporation of iron by apoferritin. Biochim Biophys Acta. 1957 Nov;26(2):441–443. doi: 10.1016/0006-3002(57)90036-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Macara I. G., Hoy T. G., Harrison P. M. The formation of ferritin from apoferritin. Kinetics and mechanism of iron uptake. Biochem J. 1972 Jan;126(1):151–162. doi: 10.1042/bj1260151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M., Stahl F. W., Vinograd J. EQUILIBRIUM SEDIMENTATION OF MACROMOLECULES IN DENSITY GRADIENTS. Proc Natl Acad Sci U S A. 1957 Jul 15;43(7):581–588. doi: 10.1073/pnas.43.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederer W. Ferritin: iron incorporation and iron release. Experientia. 1970;26(2):218–220. doi: 10.1007/BF01895596. [DOI] [PubMed] [Google Scholar]
- Pape L., Multani J. S., Stitt C., Saltman P. In vitro reconstitution of ferritin. Biochemistry. 1968 Feb;7(2):606–612. doi: 10.1021/bi00842a014. [DOI] [PubMed] [Google Scholar]