Abstract
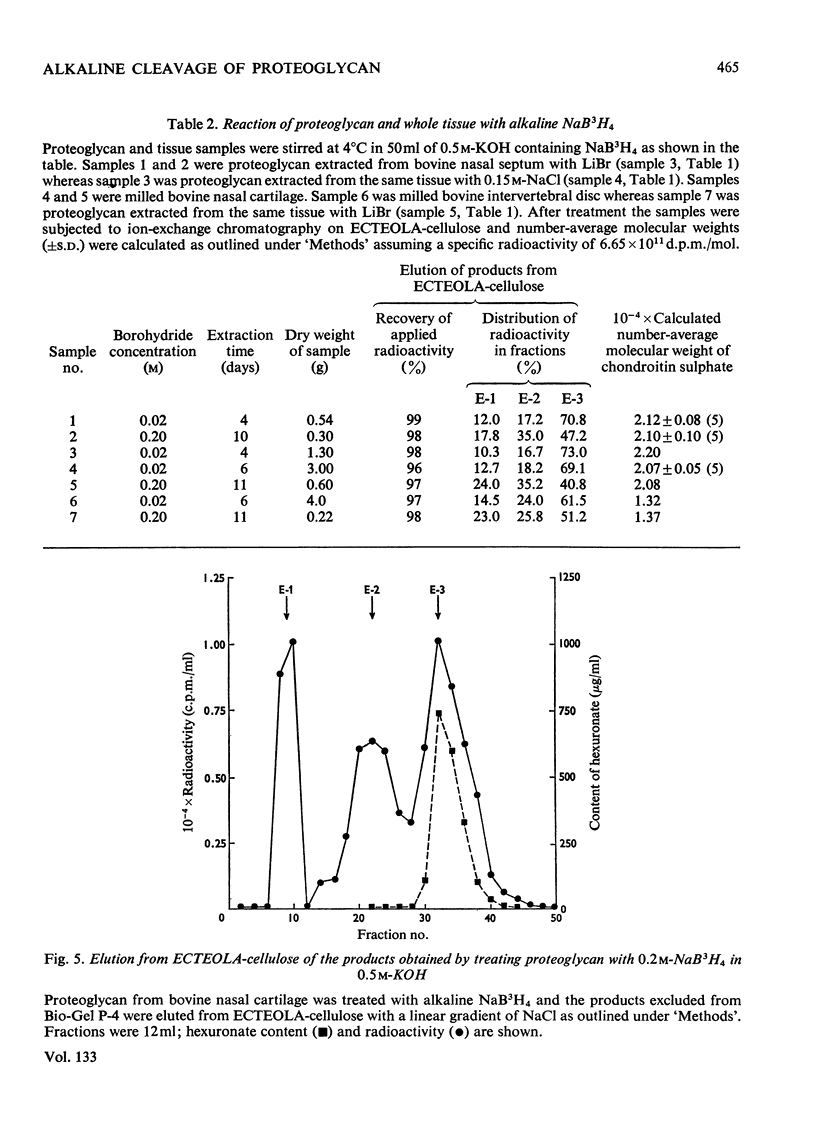
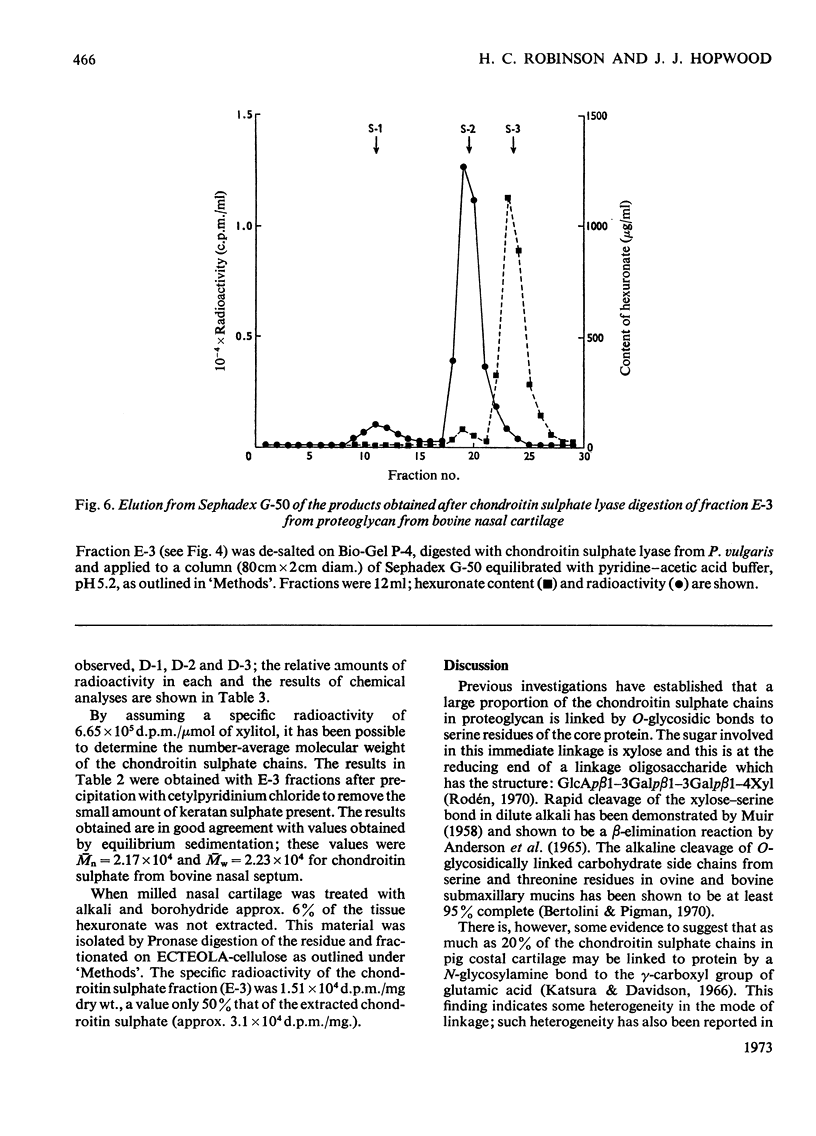
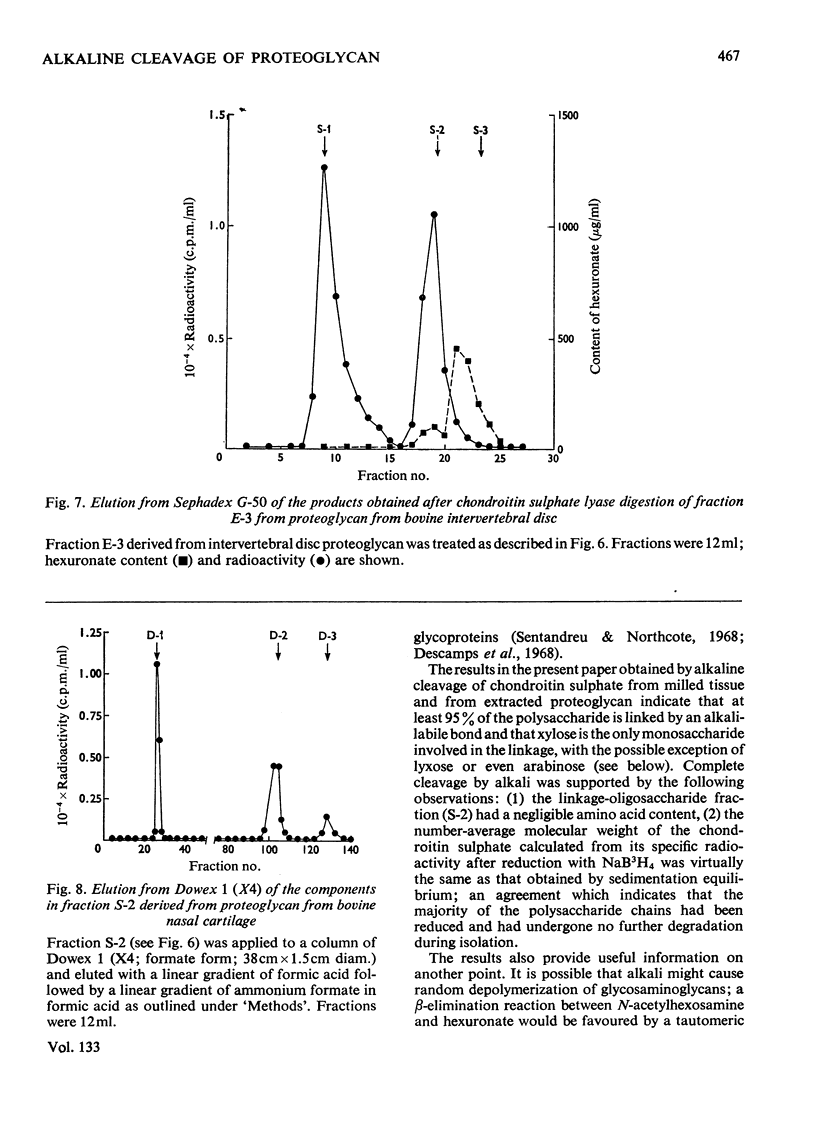
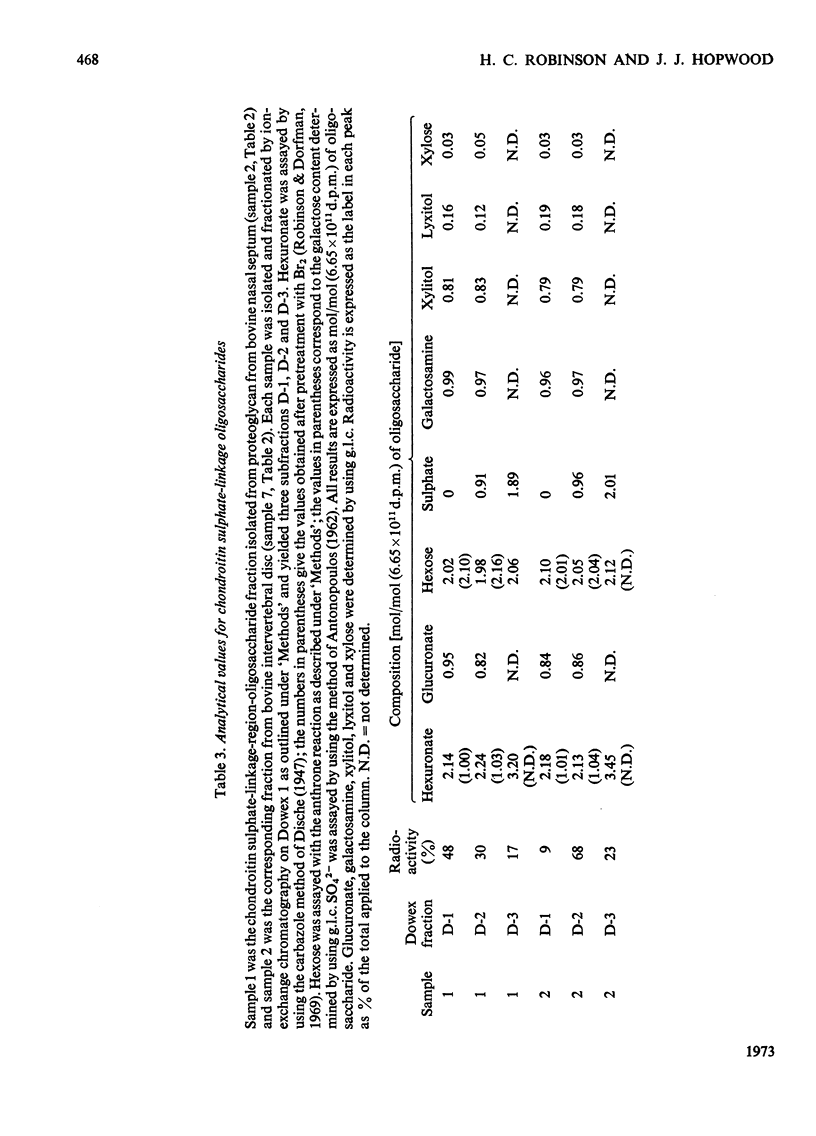
A method for the rapid isolation and purification of proteoglycan by using neutral solutions of LiBr for extraction and density-gradient centrifugation is described. The effect of 0.5m-KOH on isolated proteoglycan has been studied by using NaB3H4 to reduce and label the chondroitin sulphate chains released. This study has established: (a) that at least 95% of the chondroitin sulphate chains are attached to the proteoglycan by alkali-labile bonds between xylose and serine; (b) that random degradation of the chondroitin sulphate chains does not occur to any significant extent; (c) that the method is convenient for the determination of polysaccharide number-average molecular weights.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON B., HOFFMAN P., MEYER K. THE O-SERINE LINKAGE IN PEPTIDES OF CHONDROITIN 4- OR 6-SULFATE. J Biol Chem. 1965 Jan;240:156–167. [PubMed] [Google Scholar]
- BOAS N. F. Method for the determination of hexosamines in tissues. J Biol Chem. 1953 Oct;204(2):553–563. [PubMed] [Google Scholar]
- Buddecke E., Platt D. Untersuchungen zur Chemie der Arterienwand. 8. Nachweis, Reinigung und Eigenschaften der Hyaluronidase aus der Aorta des Rindes. Hoppe Seylers Z Physiol Chem. 1965;343(1):61–78. [PubMed] [Google Scholar]
- Clamp J. R., Dawson G., Hough L. The simultaneous estimation of 6-deoxy-L-galactose (L-fucose), D-mannose, D-galactose, 2-acetamido-2-deoxy-D-glucose (N-acetyl-D-glucosamine) and N-acetylneuraminic acid (sialic acid) in glycopeptides and glycoproteins. Biochim Biophys Acta. 1967 Nov 28;148(2):342–349. doi: 10.1016/0304-4165(67)90129-8. [DOI] [PubMed] [Google Scholar]
- Creeth J. M., Pain R. H. The determination of molecular weights of biological macromolecules by ultracentrifuge methods. Prog Biophys Mol Biol. 1967;17:217–287. doi: 10.1016/0079-6107(67)90008-9. [DOI] [PubMed] [Google Scholar]
- Descamps J., Monsigny M., Montreuil J. Etude sur les glycoprotéines. Mise en évidence de liaisons O-séryl et O-thréonyl-N-acétylgalactosaminidiques dans les globulines gamma-A du lait de femme. C R Acad Sci Hebd Seances Acad Sci D. 1968 Apr 22;266(17):1775–1778. [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Katsura N., Davidson E. A. Studies on porcine costal cartilage protein-polysaccharide complex. II. Chemical studies. Biochim Biophys Acta. 1966 May 26;121(1):128–134. [PubMed] [Google Scholar]
- LINDAHL U., RODEN L. THE ROLE OF GALACTOSE AND XYLOSE IN THE LINKAGE OF HEPARIN TO PROTEIN. J Biol Chem. 1965 Jul;240:2821–2826. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lloyd K. O., Kabat E. A., Layug E. J., Gruezo F. Immunochemical studies on blood groups. XXXIV. Structures of some oligosaccharides produced by alkaline degradation of blood group A, B, and H substances. Biochemistry. 1966 May;5(5):1489–1501. doi: 10.1021/bi00869a007. [DOI] [PubMed] [Google Scholar]
- Luscombe M., Phelps C. F. The composition and physicochemical properties of bovine nasal-septa protein-polysaccharide complex. Biochem J. 1967 Jan;102(1):110–119. doi: 10.1042/bj1020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUIR H. The nature of the link between protein and carbohydrate of a chondroitin sulphate complex from hyaline cartilage. Biochem J. 1958 Jun;69(2):195–204. doi: 10.1042/bj0690195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S., Doganges P. T., Schubert M. The separation of new forms of the proteinpolysaccharides of bovine nasal cartilage. J Biol Chem. 1966 Sep 25;241(18):4261–4266. [PubMed] [Google Scholar]
- Preston B. N. Physical characterization of dermatan sulphate-protein. Arch Biochem Biophys. 1968 Sep 10;126(3):974–977. doi: 10.1016/0003-9861(68)90500-6. [DOI] [PubMed] [Google Scholar]
- Robinson H. C., Dorfman A. The sulfation of chondroitin sulfate in embryonic chick cartilage epiphyses. J Biol Chem. 1969 Jan 25;244(2):348–352. [PubMed] [Google Scholar]
- SENO N., MEYER K., ANDERSON B., HOFFMAN P. VARIATIONS IN KERATOSULFATES. J Biol Chem. 1965 Mar;240:1005–1010. [PubMed] [Google Scholar]
- SPECK J. C., Jr The Lobry de Bruyn-Alberda van Ekenstein transformation. Adv Carbohydr Chem. 1958;13:63–103. doi: 10.1016/s0096-5332(08)60352-5. [DOI] [PubMed] [Google Scholar]
- Sajdera S. W., Hascall V. C. Proteinpolysaccharide complex from bovine nasal cartilage. A comparison of low and high shear extraction procedures. J Biol Chem. 1969 Jan 10;244(1):77–87. [PubMed] [Google Scholar]
- Sentandreu R., Northcote D. H. The structure of a glycopeptide isolated from the yeast cell wall. Biochem J. 1968 Sep;109(3):419–432. doi: 10.1042/bj1090419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. I. Fractionation and microdetermination of cell carbohydrates. Biochem J. 1952 Jan;50(3):298–303. doi: 10.1042/bj0500298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiganos C. P., Muir H. Studies on protein-polysaccharides from pig laryngeal cartilage. Heterogeneity, fractionation and characterization. Biochem J. 1969 Aug;113(5):885–894. doi: 10.1042/bj1130885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]


