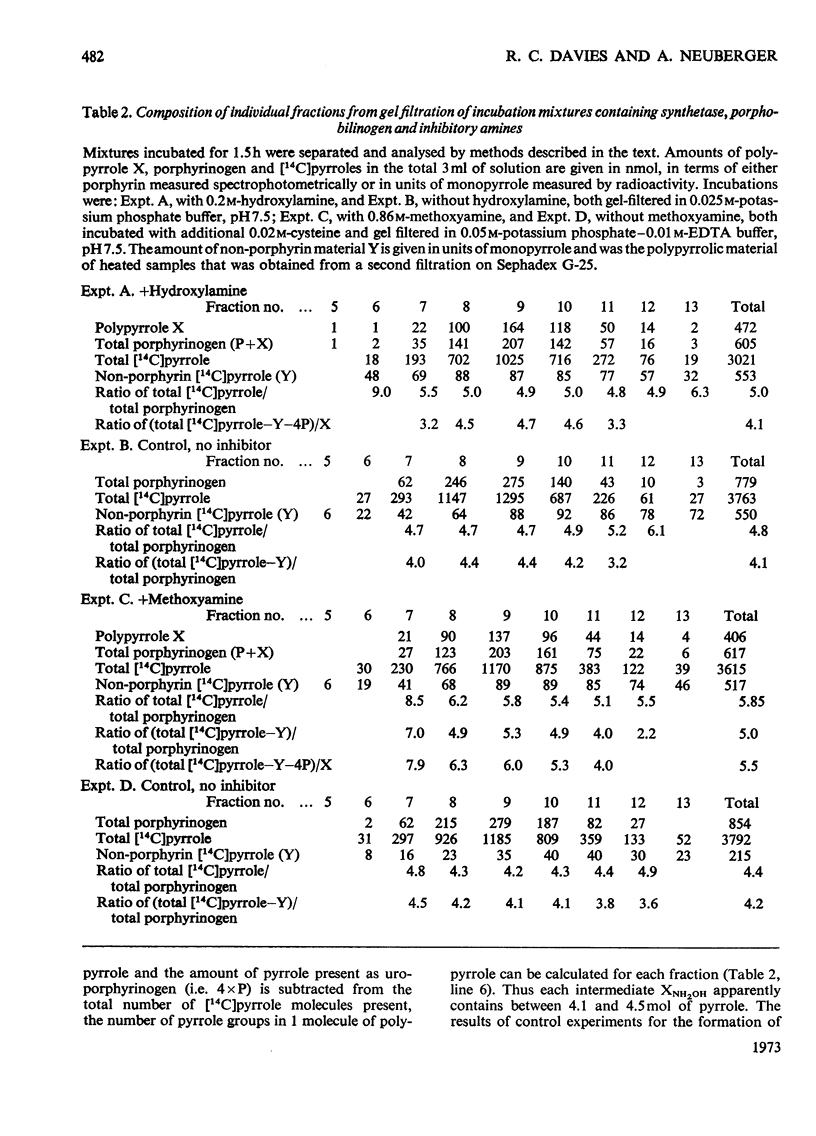
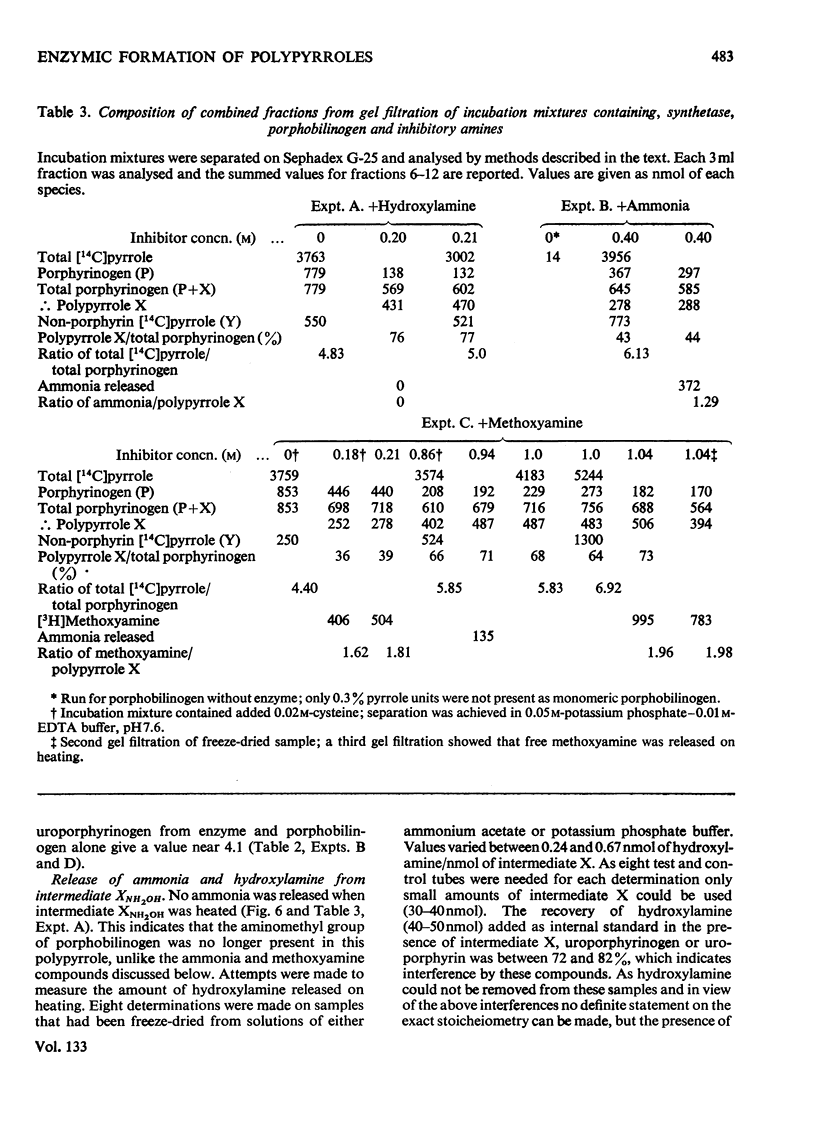
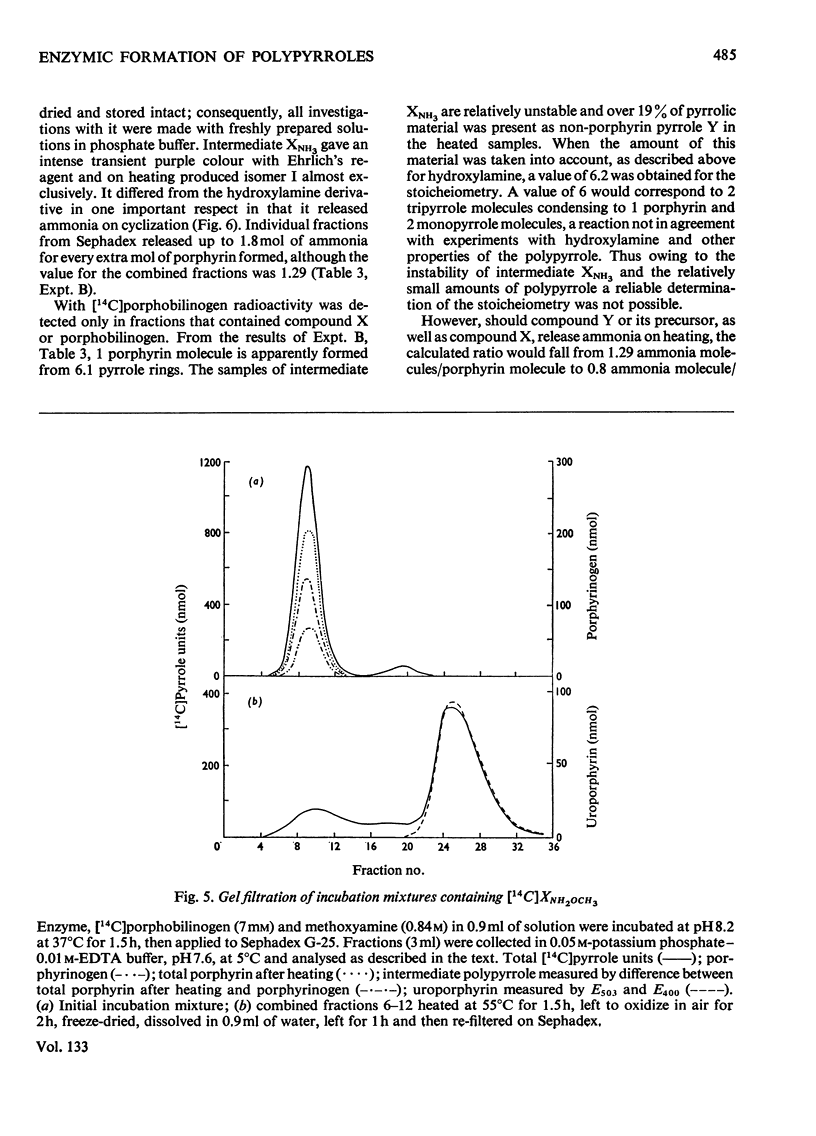
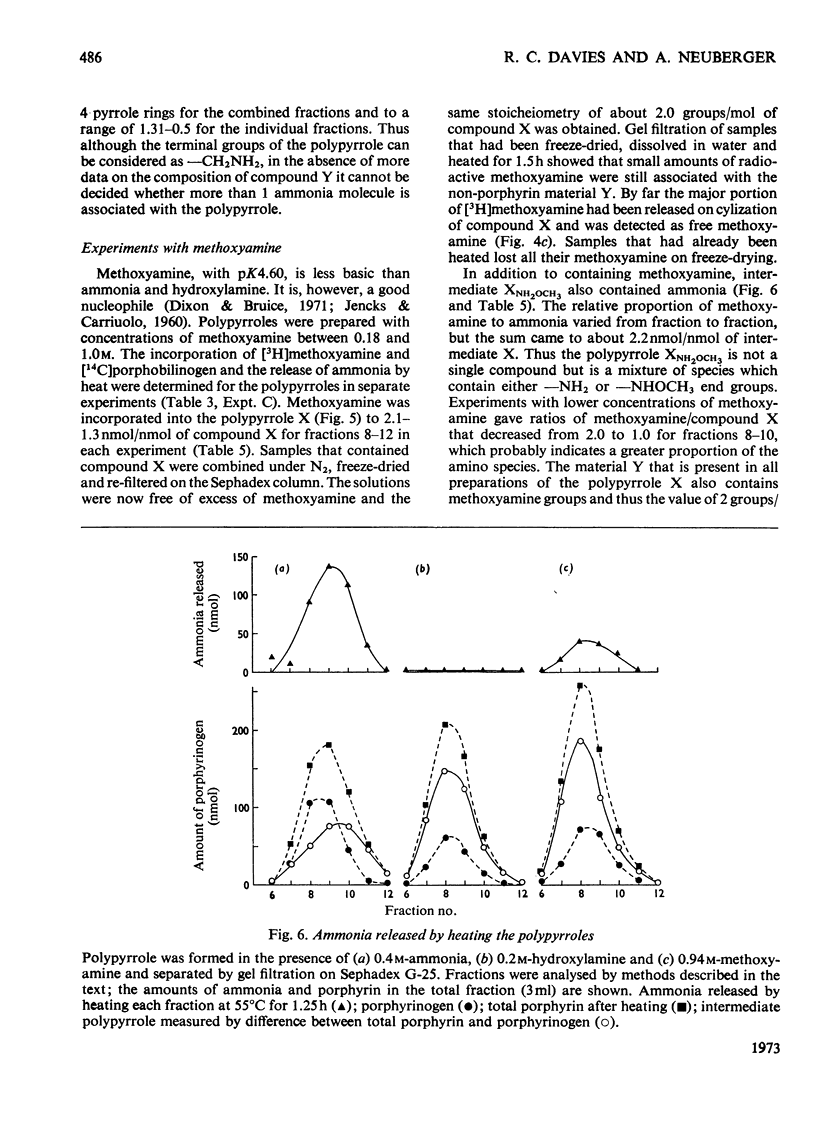
Abstract
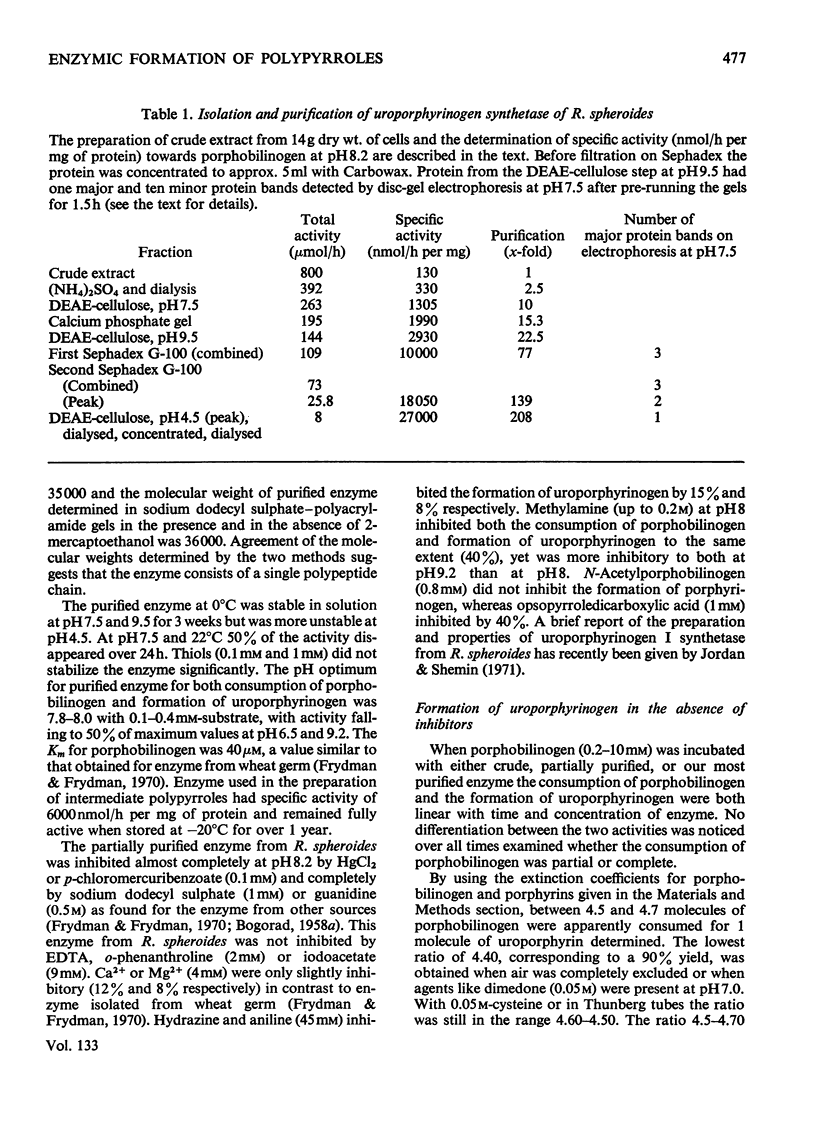
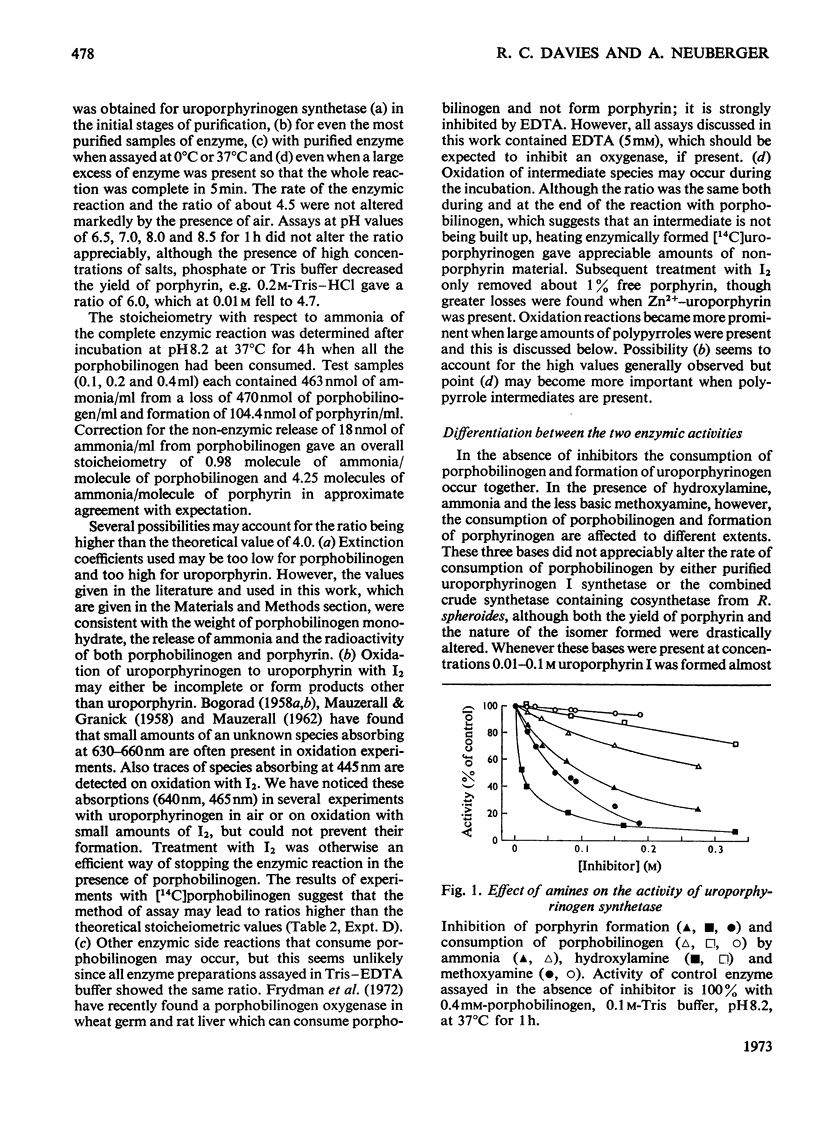
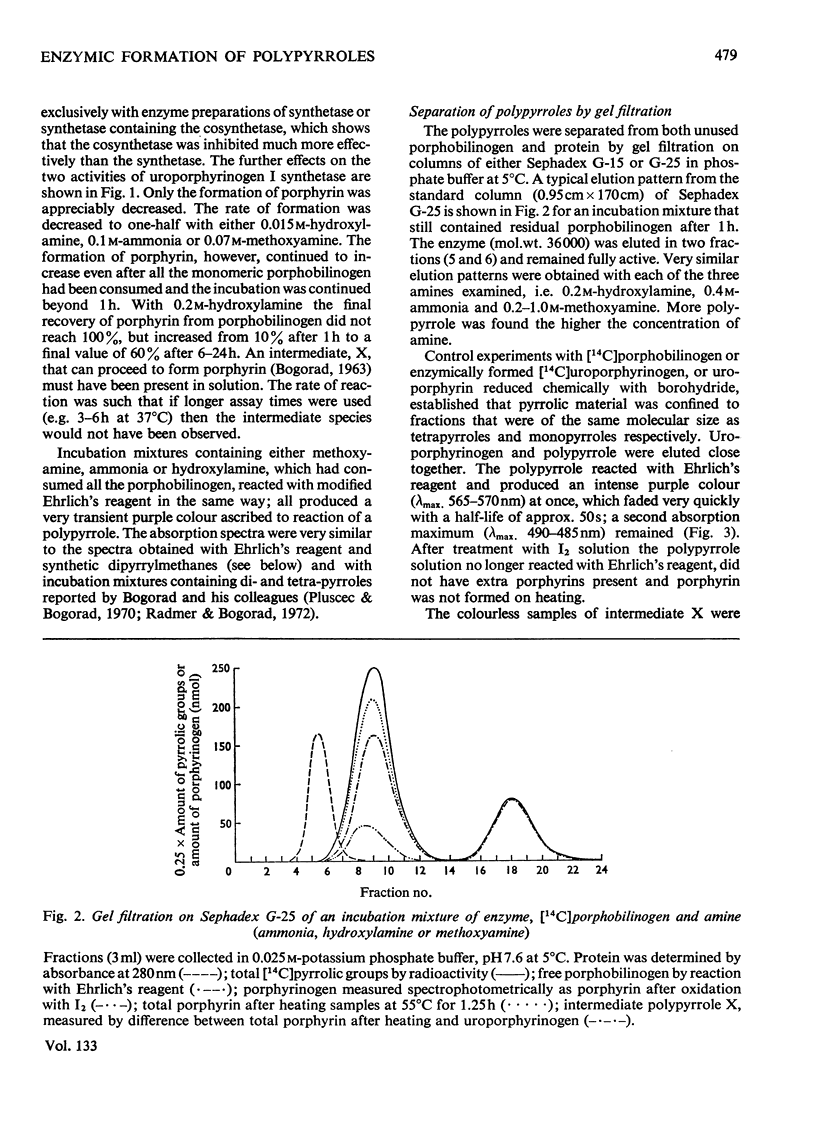
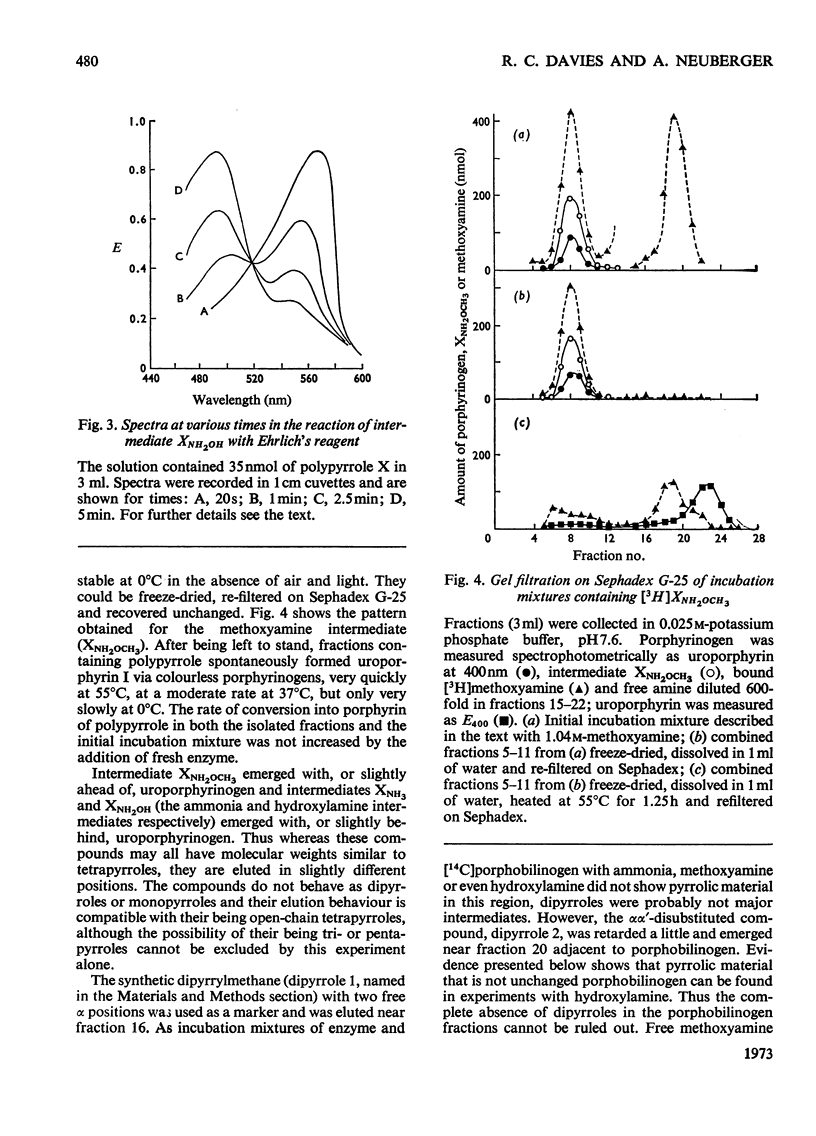
1. Uroporphyrinogen I synthetase of Rhodopseudomonas spheroides was purified more than 200-fold from the soluble protein of broken bacterial cells. The enzyme had molecular weight 36000, an isoelectric point of 4.46 and migrated as a single active protein band on disc-gel electrophoresis at pH7.5 and 8.9. 2. The enzyme consumed porphobilinogen and formed uroporphyrinogen at pH8.2 without the accumulation of intermediates. In the presence of hydroxylamine, ammonia or methoxyamine the production of porphyrinogen was inhibited and the enzyme formed open-chain polypyrroles instead. 3. These polypyrroles behaved like uroporphyrinogen on Sephadex G-25; they were colourless and had unsubstituted α-pyrrolic positions. The inhibitory amines were incorporated into the molecules. 4. The polypyrroles formed porphyrins non-enzymically and the cyclization reaction was accompanied by the release of the inhibitory amine. Exchange of the amino function of the original porphobilinogen in the polypyrrole was complete with hydroxylamine and almost complete with methoxyamine, both ammonia and methoxyamine being present in the polypyrrolic material. 5. The behaviour, properties and composition of the radioactive hydroxylamine derivative were consistent with a tetrapyrrolic structure, probably a pyrrylmethane, that was not cyclized, rather than with di-, tri- or penta-pyrrolic structures. No monopyrrolic or dipyrrolic Ehrlich-positive material was released on cyclization. The ammonia and methoxyamine derivatives had properties similar to the hydroxylamine derivative. 6. Another modified pyrrole was detected only in experiments with hydroxylamine. It differed from both porphobilinogen and known dipyrroles and appeared to be a monopyrrole. 7. The participation of positively charged reaction centres in the enzymic mechanism, particularly in the cyclization step, is discussed.
Full text
PDF








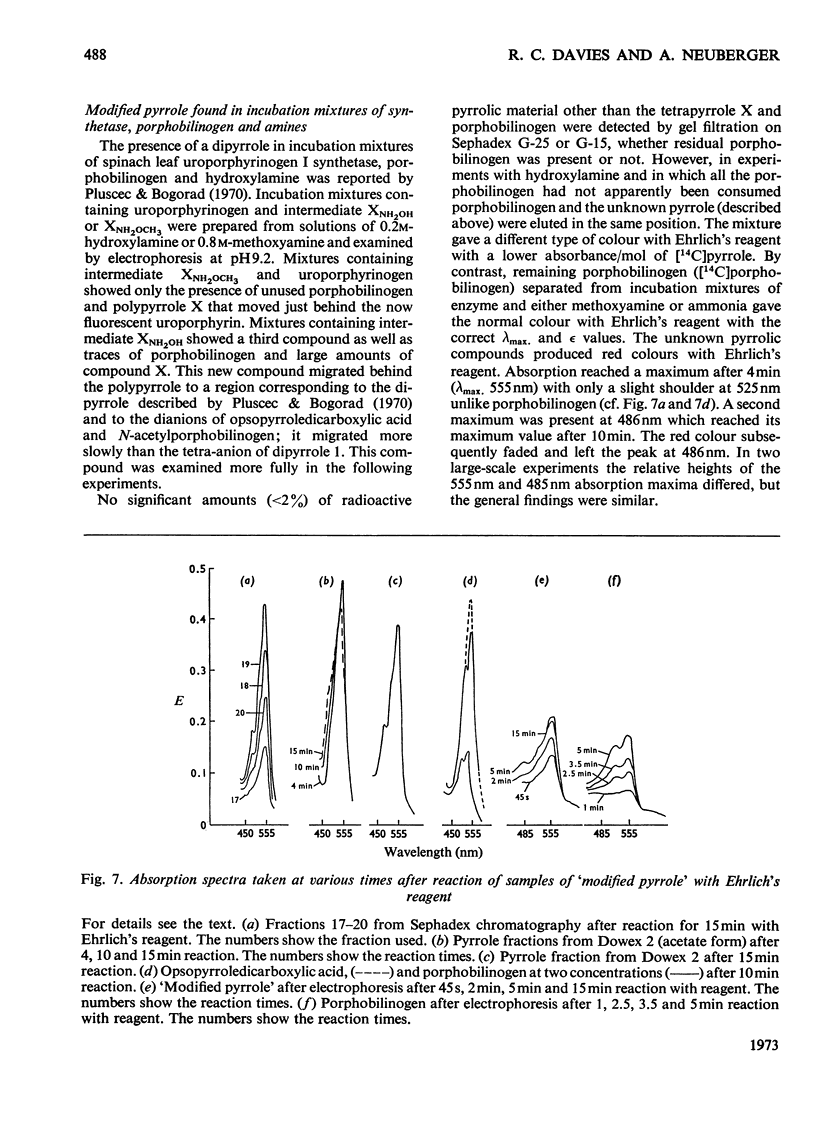

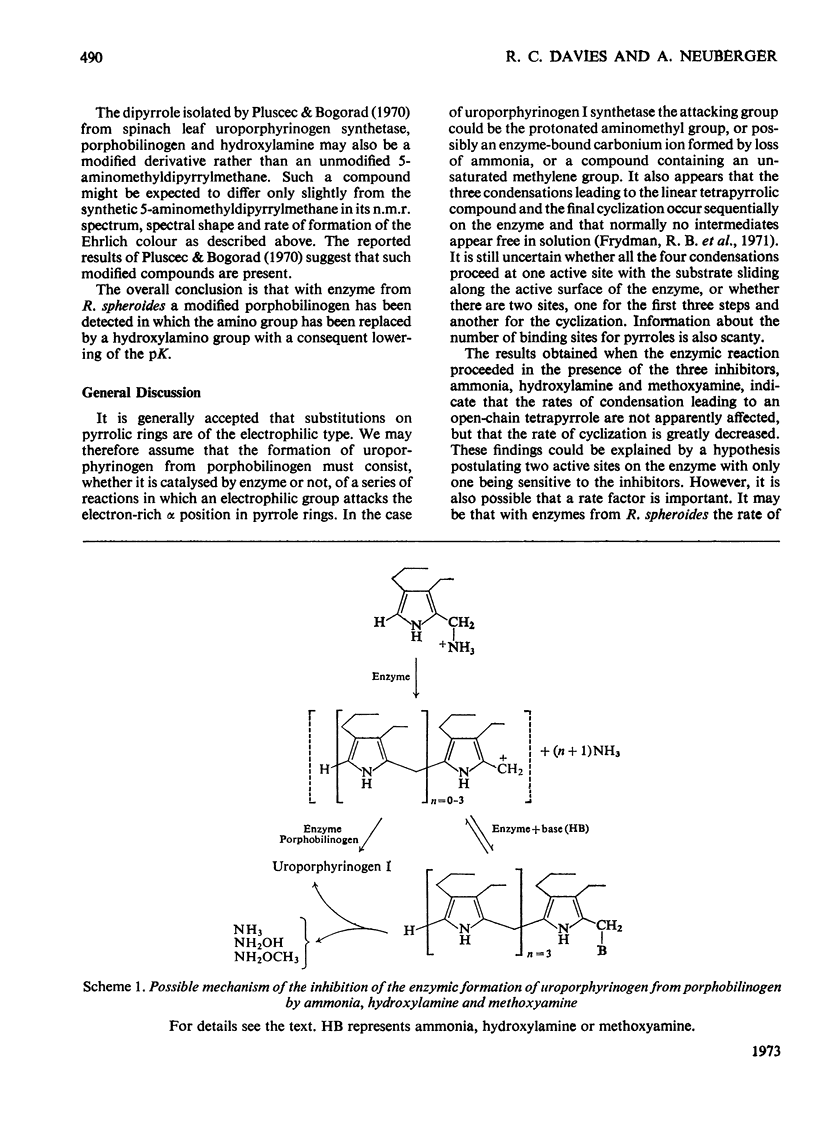


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOGORAD L. The enzymatic synthesis of porphyrins from porphobilinogen. I. Uroporphyrin I. J Biol Chem. 1958 Aug;233(2):501–509. [PubMed] [Google Scholar]
- BOGORAD L. The enzymatic synthesis of porphyrins from porphobilinogen. II. Uroporphyrin III. J Biol Chem. 1958 Aug;233(2):510–515. [PubMed] [Google Scholar]
- BURNHAM B. F., PIERCE W. S., WILLIAMS K. R., BOYER M. H., KIRBY C. K. delta-aminolaevulate dehydratase from Rhodopseudomonas spheroides. Biochem J. 1963 Jun;87:462–472. doi: 10.1042/bj0870462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON R. M., KAPLAN N. O. A chemical reaction of hydroxylamine with diphospyridine nucleotide. J Biol Chem. 1954 Nov;211(1):447–463. [PubMed] [Google Scholar]
- Budowsky E. I., Sverdlov E. D., Shibaeva R. P., Monastyrskaya G. S., Kochetkov N. K. Mechanism of the mutagenic action of hydroxylamine. 3. Reaction of hydroxylamine and O-methylhydroxylamine with the cytosine nucleus. Biochim Biophys Acta. 1971 Aug 26;246(2):300–319. [PubMed] [Google Scholar]
- Budowsky E. I., Turchinsky M. F., Domkin V. D., Pogorelov A. G., Pisarenko V. N., Kusova K. S., Kochetkov N. K. Mechanism of the mutagenic action of hydroxylamine. VI. Reaction of hydroxylamine with the uracil nucleus. Biochim Biophys Acta. 1972 Aug 25;277(2):421–437. doi: 10.1016/0005-2787(72)90422-4. [DOI] [PubMed] [Google Scholar]
- CHANEY A. L., MARBACH E. P. Modified reagents for determination of urea and ammonia. Clin Chem. 1962 Apr;8:130–132. [PubMed] [Google Scholar]
- COOKSON G. H., RIMINGTON C. Porphobilinogen. Biochem J. 1954 Jul;57(3):476–484. doi: 10.1042/bj0570476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORNFORD P. A., BENSON A. A qualitative and quantitative study of the separation of uroporphyrin octamethyl esters I and III by dioxan chromatography. J Chromatogr. 1963 Feb;10:141–157. doi: 10.1016/s0021-9673(01)92287-3. [DOI] [PubMed] [Google Scholar]
- Cornford P. Transformation of porphobilinogen into porphyrins by preparations from human erythrocytes. Biochem J. 1964 Apr;91(1):64–73. doi: 10.1042/bj0910064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton J., Dougherty R. C. Formation of the macrocyclic ring in tetrapyrrole biosynthesis. Nature. 1969 Sep 13;223(5211):1151–1153. doi: 10.1038/2231151a0. [DOI] [PubMed] [Google Scholar]
- Frydman R. B., Frydman B. Purification and properties of porphobilinogen deaminase from wheat germ. Arch Biochem Biophys. 1970 Jan;136(1):193–202. doi: 10.1016/0003-9861(70)90341-3. [DOI] [PubMed] [Google Scholar]
- Frydman R. B., Reil S., Frydman B. Relation between structure and reactivity in porphobilinogen and related pyrroles. Biochemistry. 1971 Mar 30;10(7):1154–1160. doi: 10.1021/bi00783a009. [DOI] [PubMed] [Google Scholar]
- Frydman R. B., Tomaro M. L., Wanschelbaum A., Frydman B. The enzymatic oxidation of porphobilinogen. FEBS Lett. 1972 Oct 1;26(1):203–206. doi: 10.1016/0014-5793(72)80573-8. [DOI] [PubMed] [Google Scholar]
- HOARE D. S., HEATH H. The biosynthesis of porphyrins from porphobilinogen by Rhodopseudomonas spheroides. Biochem J. 1959 Dec;73:679–690. doi: 10.1042/bj0730679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASCELLES J. The synthesis of porphyrins and bacteriochlorophyll by cell suspensions of Rhodopseudomonas spheroides. Biochem J. 1956 Jan;62(1):78–93. doi: 10.1042/bj0620078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin E. Y., Coleman D. L. The enzymatic conversion of porphobilinogen to uroporphyrinogen catalyzed by extracts of hematopoietic mouse spleen. J Biol Chem. 1967 Sep 25;242(18):4247–4253. [PubMed] [Google Scholar]
- Llambias E. B.C., del C Batlle A. M. Uroporphyrinogen III cosynthetase. Evidence for the existence of a polypyrrolic substrate in soybean callus tissue. FEBS Lett. 1970 Feb 16;6(3):285–288. doi: 10.1016/0014-5793(70)80079-5. [DOI] [PubMed] [Google Scholar]
- Llambías E. B., Batlle A. M. Porphyrin biosynthesis. 8. Avian erythrocyte porphobilinogen deaminase-uroporphyrinogen 3 cosynthetase, its purification, properties and the separation of its components. Biochim Biophys Acta. 1971 Jan 13;227(1):180–191. doi: 10.1016/0005-2744(71)90178-1. [DOI] [PubMed] [Google Scholar]
- Llambías E. B., Battle A. M. Studies on the porphobilinogen deaminase-uroporphyrinogen cosynthetase system of cultured soya-bean cells. Biochem J. 1971 Jan;121(2):327–340. doi: 10.1042/bj1210327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. Porphyrin biosynthesis in erythrocytes. III. Uroporphyrinogen and its decarboxylase. J Biol Chem. 1958 Jun;232(2):1141–1162. [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- Nandi D. L., Baker-Cohen K. F., Shemin D. Delta-aminolevulinic acid dehydratase of Rhodopseudomonas spheroides. J Biol Chem. 1968 Mar 25;243(6):1224–1230. [PubMed] [Google Scholar]
- Pluscec J., Bogorad L. A dipyrrylmethane intermediate in the enzymatic synthesis of uroporphyrinogen. Biochemistry. 1970 Nov 24;9(24):4736–4743. doi: 10.1021/bi00826a017. [DOI] [PubMed] [Google Scholar]
- Radmer R., Bogorad L. A tetrapyrrylmethane intermediate in the enzymatic synthesis of uroporphyrinogen. Biochemistry. 1972 Feb 29;11(5):904–910. doi: 10.1021/bi00755a033. [DOI] [PubMed] [Google Scholar]
- Rimington C. Spectral-absorption coefficients of some porphyrins in the Soret-band region. Biochem J. 1960 Jun;75(3):620–623. doi: 10.1042/bj0750620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEIFTER S., GALLOP P. M., MICHAELS S., MEILMAN E. Analysis of hydroxamic acids and hydrazides; preparation and properties of dinitrophenyl derivatives of hydroxamic acids, oximes, hydrazides, and hydrazones. J Biol Chem. 1960 Sep;235:2613–2618. [PubMed] [Google Scholar]
- Stella A. M., Parera V. E., Llambías E. B., Batlle A. M. Pyrrylmethane intermediates in the synthesis of uroporphyrinogen 3 by soybean callus porphobilinogenase. Biochim Biophys Acta. 1971 Dec 21;252(3):481–488. doi: 10.1016/0304-4165(71)90151-6. [DOI] [PubMed] [Google Scholar]
- Tait G. H. Glycine decarboxylase in Rhodopseudomonas spheroides and in rat liver mitochondria. Biochem J. 1970 Aug;118(5):819–830. doi: 10.1042/bj1180819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WESTALL R. G. Isolation of porphobilinogen from the urine of a patient with acute porphyria. Nature. 1952 Oct 11;170(4328):614–616. doi: 10.1038/170614a0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


