Abstract
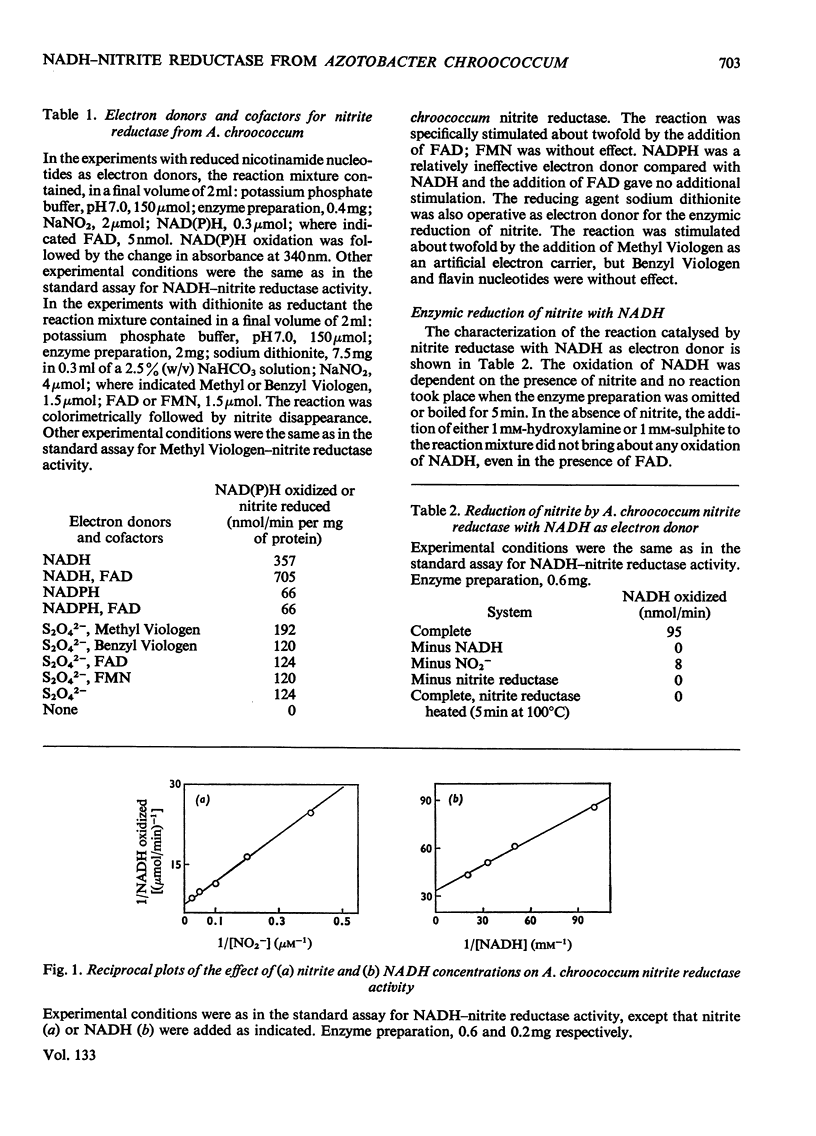
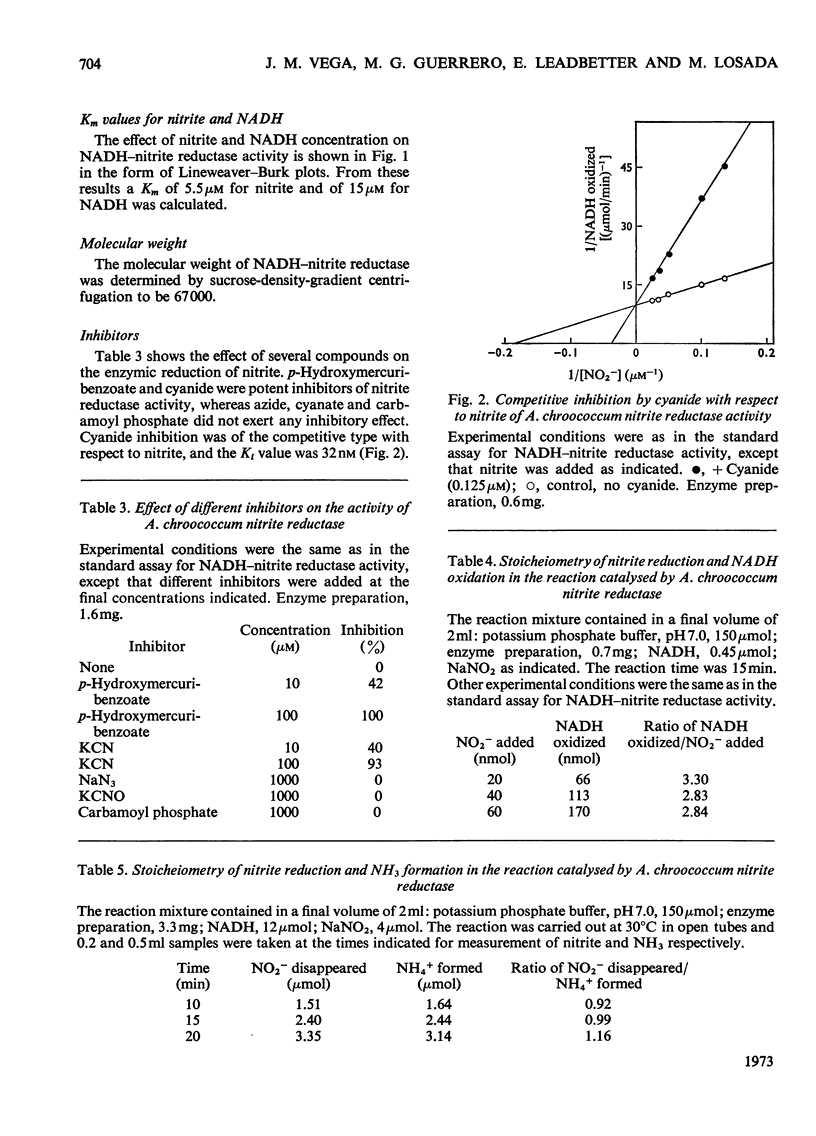
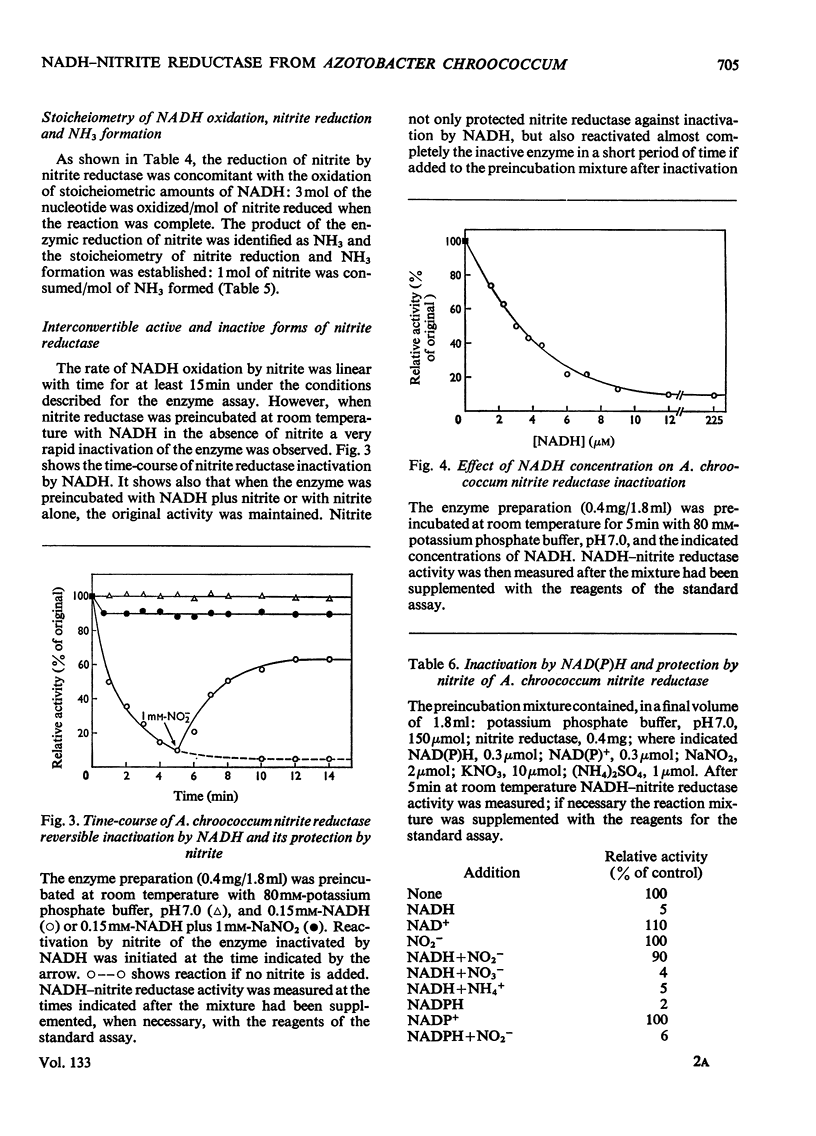
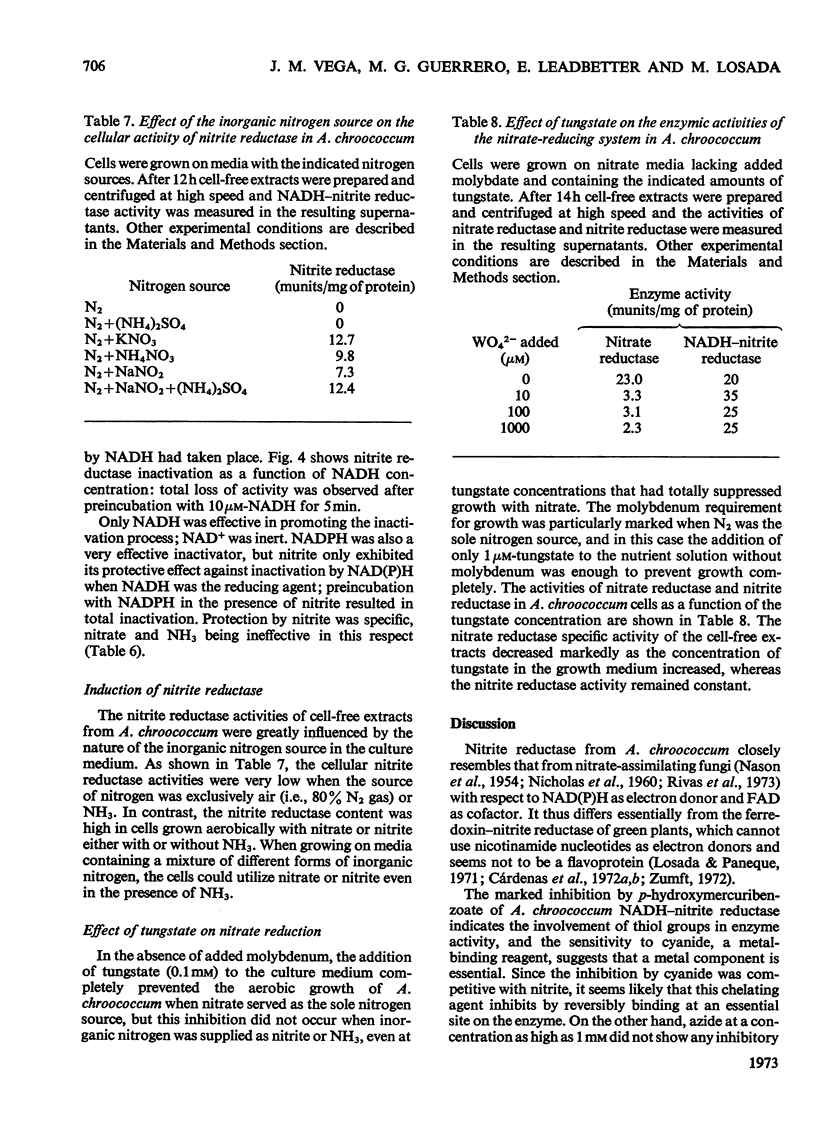
1. The assimilatory nitrite reductase of the N2-fixing bacterium Azotobacter chroococcum was prepared in a soluble form from cells grown aerobically with nitrate as the nitrogen source, and some of its properties have been studied. 2. The enzyme is a FAD-dependent metalloprotein (mol.wt. about 67000), which stoicheiometrically catalyses the direct reduction of nitrite to NH3 with NADH as the electron donor. 3. NADH–nitrite reductase can exist in two either active or inactive interconvertible forms. Inactivation in vitro can be achieved by preincubation with NADH. Nitrite can specifically protect the enzyme against this inactivation and reverse the process once it has occurred. 4. A. chroococcum nitrite reductase is an adaptive enzyme whose formation depends on the presence of either nitrate or nitrite in the nutrient solution. 5. Tungstate inhibits growth of the microorganism very efficiently, by competition with molybdate, when nitrate is the nitrogen source, but does not interfere when nitrite or NH3 is substituted for nitrate. The addition of tungstate to the culture media results in the loss of nitrate reductase activity but does not affect nitrite reductase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cárdenas J., Barea J. L., Rivas J., Moreno C. G. Purification and properties of nitrite reductase from spinach leaves. FEBS Lett. 1972 Jun 15;23(2):131–135. doi: 10.1016/0014-5793(72)80322-3. [DOI] [PubMed] [Google Scholar]
- Herrera J., Paneque A., Maldonado J. M., Barea J. L., Losada M. Regulation by ammonia of nitrate reductase synthesis and activity in Chlamydomonas reinhardi. Biochem Biophys Res Commun. 1972 Aug 21;48(4):996–1003. doi: 10.1016/0006-291x(72)90707-3. [DOI] [PubMed] [Google Scholar]
- Jetschmann K., Solomonson L. P., Vennesland B. Activation of nitrate reductase by oxidation. Biochim Biophys Acta. 1972 Aug 17;275(2):276–278. doi: 10.1016/0005-2728(72)90048-5. [DOI] [PubMed] [Google Scholar]
- Kemp J. D., Atkinson D. E. Nitrite reductase of Escherichia coli specific for reduced nicotinamide adenine dinucleotide. J Bacteriol. 1966 Sep;92(3):628–634. doi: 10.1128/jb.92.3.628-634.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAZZARINI R. A., ATKINSON D. E. A triphosphopyridine nucleotide-specific nitrite reductase from Escherichia coli. J Biol Chem. 1961 Dec;236:3330–3335. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Losada M., Paneque A., Aparicio P. J., Vega J. M., Cárdenas J., Herrera J. Inactivation and repression by ammonium of the nitrate reducing system in chlorella. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1009–1015. doi: 10.1016/0006-291x(70)90340-2. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Maldonado J. M., Herrera J., Paneque A., Losada M. Reversible inactivation by NADH and ADP on Chlorella fusca nitrate reductase. Biochem Biophys Res Commun. 1973 Mar 5;51(1):27–33. doi: 10.1016/0006-291x(73)90502-0. [DOI] [PubMed] [Google Scholar]
- Moreno C. G., Aparicio P. J., Palacián E., Losada M. Interconversion of the active and inactive forms of Chlorella nitrate reductase. FEBS Lett. 1972 Oct 1;26(1):11–14. doi: 10.1016/0014-5793(72)80530-1. [DOI] [PubMed] [Google Scholar]
- NASON A., ABRAHAM R. G., AVERBACH B. C. The enzymic reduction of nitrite to ammonia by reduced pyridine nucleotides. Biochim Biophys Acta. 1954 Sep;15(1):159–161. doi: 10.1016/0006-3002(54)90118-3. [DOI] [PubMed] [Google Scholar]
- NASON A. Symposium on metabolism of inorganic compounds. II. Enzymatic pathways of nitrate, nitrite, and hydroxylamine metabolisms. Bacteriol Rev. 1962 Mar;26:16–41. doi: 10.1128/br.26.1.16-41.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHOLAS D. J., MEDINA A., JONES O. T. A nitrite reductase from Neurospora crassa. Biochim Biophys Acta. 1960 Jan 29;37:468–476. doi: 10.1016/0006-3002(60)90503-5. [DOI] [PubMed] [Google Scholar]
- Pichinoty F., Méténier G. Contribution a l'étude de la nitrate-réductase assimilatrice d'une levure. Ann Inst Pasteur (Paris) 1966 Sep;111(3):282–313. [PubMed] [Google Scholar]
- Prakash O. M., Sadana J. C. Purification, characterization and properties of nitrite reductase of Achromobacter fischeri. Arch Biochem Biophys. 1972 Feb;148(2):614–632. doi: 10.1016/0003-9861(72)90181-6. [DOI] [PubMed] [Google Scholar]
- Ramírez J. M., Del Campo F. F., Paneque A., Losada M. Ferredoxin-nitrite reductase from spinach. Biochim Biophys Acta. 1966 Apr 12;118(1):58–71. doi: 10.1016/s0926-6593(66)80144-3. [DOI] [PubMed] [Google Scholar]
- SPENCER D., TAKAHASHI H., NASON A. Relationship of nitrite and hydroxylamine reductases to nitrate assimilation and nitrogen fixation in Azotobacter agile. J Bacteriol. 1957 Apr;73(4):553–562. doi: 10.1128/jb.73.4.553-562.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega J. M., Herrera J., Aparicio P. J., Paneque A., Losada M. Role of molybdenum in nitrate reduction by chlorella. Plant Physiol. 1971 Sep;48(3):294–299. doi: 10.1104/pp.48.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumft W. G. Ferredoxin:nitrite oxidoreductase from Chlorella. Purification and properties. Biochim Biophys Acta. 1972 Aug 28;276(2):363–375. doi: 10.1016/0005-2744(72)90996-5. [DOI] [PubMed] [Google Scholar]


