Abstract
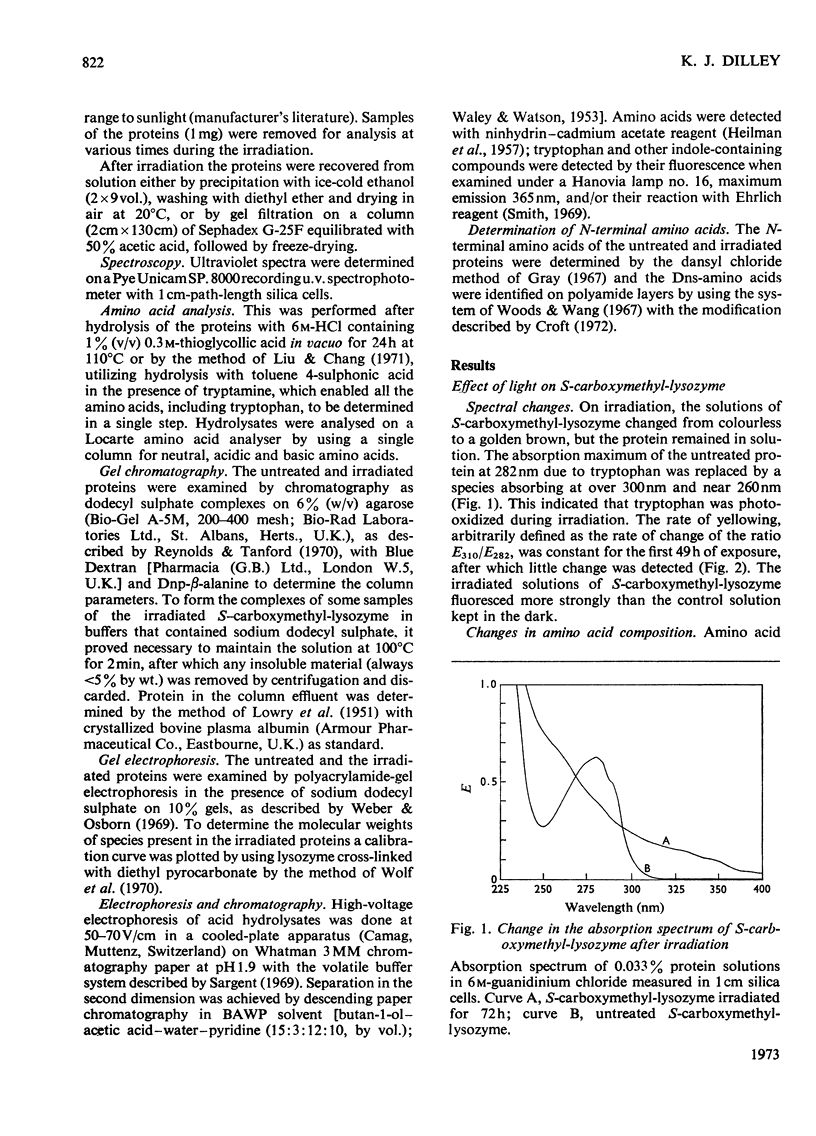
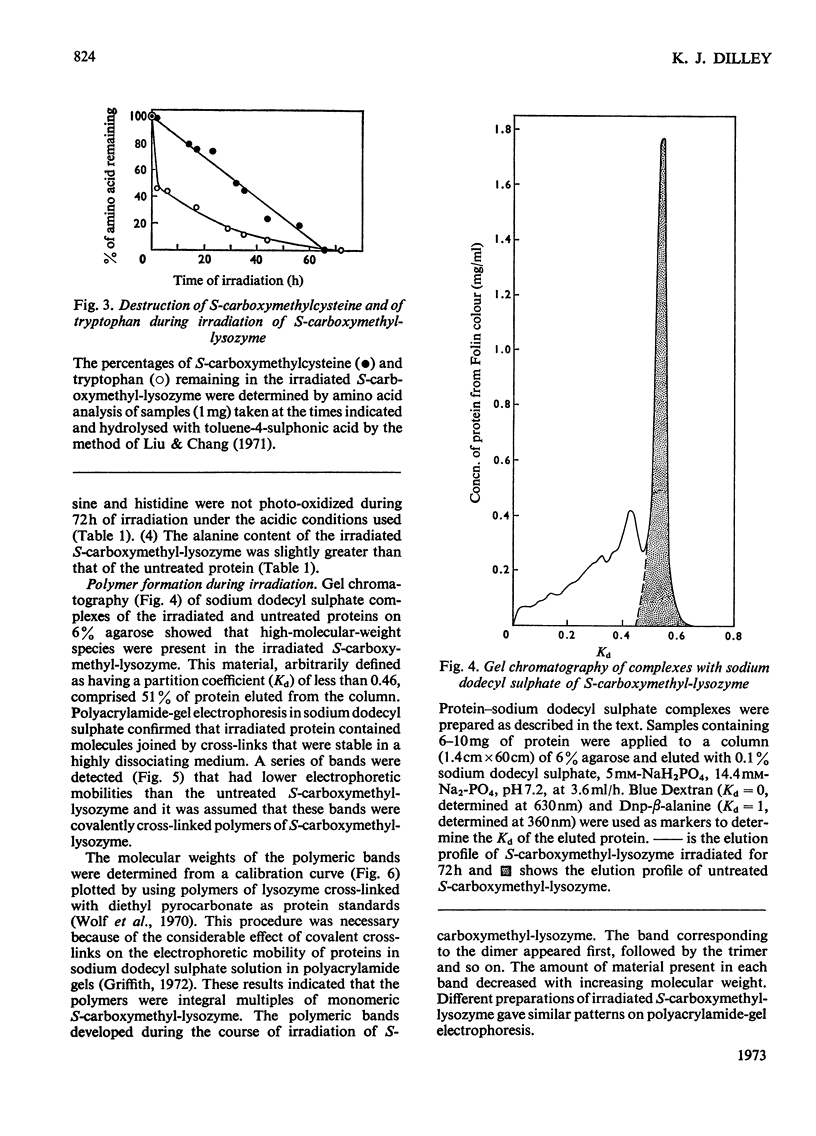
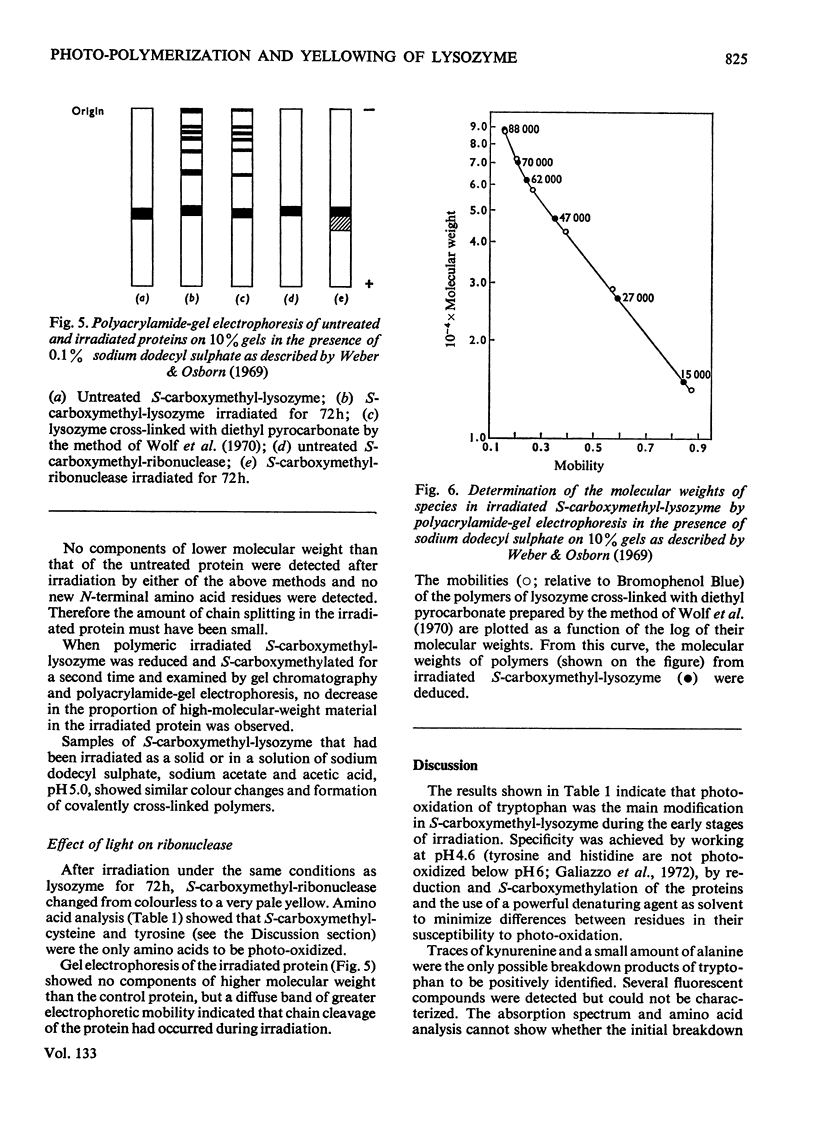
S-Carboxymethyl-lysozyme and S-carboxymethyl-ribonuclease A were irradiated with light of wavelength greater than 300nm. Photo-oxidative loss of tryptophan from the S-carboxymethyl-lysozyme was accompanied by yellowing of the protein and the formation of covalently cross-linked polymers. S-Carboxymethyl-ribonuclease, which contains no tryptophan, showed little yellowing and no polymer formation. The irradiated S-carboxymethyl-lysozyme was similar to the proteins of the brown cataractous human lens nucleus and to bovine lens proteins exposed to sunlight in vitro in that it (1) was insoluble in non-denaturing solvents, (2) contained a new fluorescence, (3) was brown in colour, and (4) contained covalent cross-links that are not disulphide bonds.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckingham R. H., Pirie A. The effect of light on lens proteins in vitro. Exp Eye Res. 1972 Nov;14(3):297–299. doi: 10.1016/0014-4835(72)90020-6. [DOI] [PubMed] [Google Scholar]
- Buckingham R. H. The behaviour of reduced proteins from normal and cataractous lenses in highly dissociating media: cross-linked protein in cataractous lenses. Exp Eye Res. 1972 Sep;14(2):123–129. doi: 10.1016/0014-4835(72)90057-7. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Croft L. R. The amino acid sequence of -crystallin (fraction II) from calf lens. Biochem J. 1972 Jul;128(4):961–970. doi: 10.1042/bj1280961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish W. W., Reynolds J. A., Tanford C. Gel chromatography of proteins in denaturing solvents. Comparison between sodium dodecyl sulfate and guanidine hydrochloride as denaturants. J Biol Chem. 1970 Oct 10;245(19):5166–5168. [PubMed] [Google Scholar]
- Griffith I. P. The effect of cross-links on the mobility of proteins in dodecyl sulphate-polyacrylamide gels. Biochem J. 1972 Feb;126(3):553–560. doi: 10.1042/bj1260553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- Harding J. J. Conformational changes in human lens proteins in cataract. Biochem J. 1972 Aug;129(1):97–100. doi: 10.1042/bj1290097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lennox F. G., Rowlands R. J. Photochemical degradation of keratins. Photochem Photobiol. 1969 Apr;9(4):359–367. doi: 10.1111/j.1751-1097.1969.tb07300.x. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Chang Y. H. Hydrolysis of proteins with p-toluenesulfonic acid. Determination of tryptophan. J Biol Chem. 1971 May 10;246(9):2842–2848. [PubMed] [Google Scholar]
- Marciani D. J., Tolbert B. M. Analytical studies of fractions from irradiated lysozyme. Biochim Biophys Acta. 1972 Jul 21;271(2):262–273. doi: 10.1016/0005-2795(72)90199-7. [DOI] [PubMed] [Google Scholar]
- Pirie A. Color and solubility of the proteins of human cataracts. Invest Ophthalmol. 1968 Dec;7(6):634–650. [PubMed] [Google Scholar]
- Pirie A. Formation of N'-formylkynurenine in proteins from lens and other sources by exposure to sunlight. Biochem J. 1971 Nov;125(1):203–208. doi: 10.1042/bj1250203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirie A. Photo-oxidation of proteins and comparison of photo-oxidized proteins with those of the cataractous human lens. Isr J Med Sci. 1972 Aug-Sep;8(8):1567–1573. [PubMed] [Google Scholar]
- WALEY S. G., WATSON J. The action of trypsin on polylysine. Biochem J. 1953 Sep;55(2):328–337. doi: 10.1042/bj0550328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wolf B., Lausarot P. M., Lesnaw J. A., Reichmann M. E. Preparation of polymeric protein markers and an investigation of their behavior in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochim Biophys Acta. 1970 Jan 20;200(1):180–183. doi: 10.1016/0005-2795(70)90060-7. [DOI] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]


